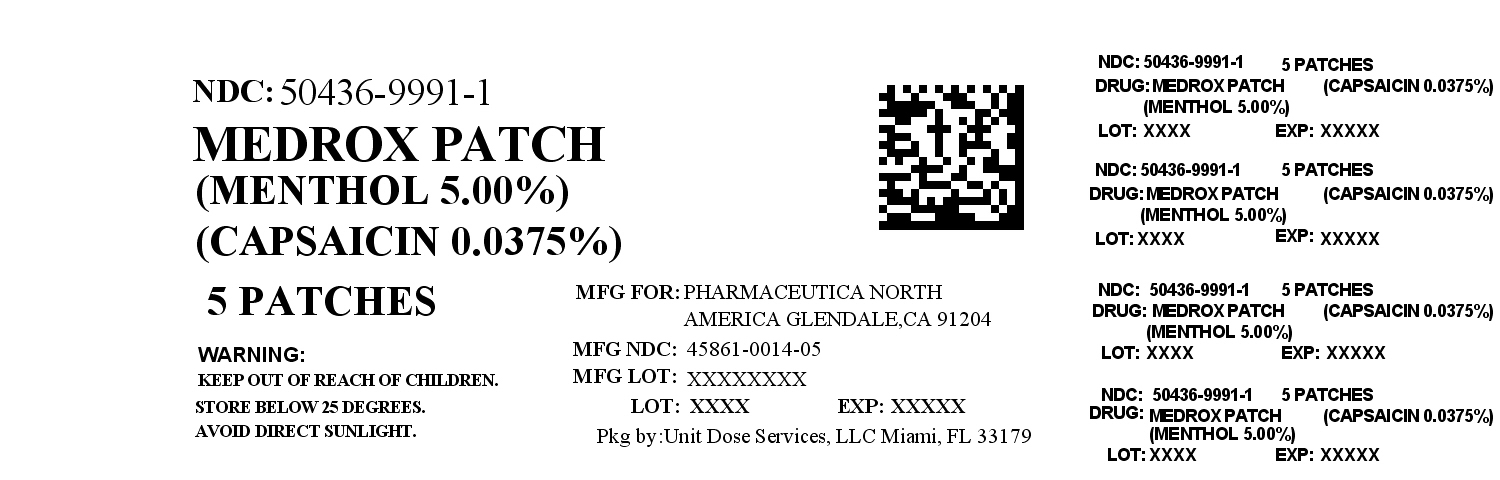
Label: MEDROX- menthol, capsaicin patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 50436-9991-1 - Packager: Unit Dose Services
- This is a repackaged label.
- Source NDC Code(s): 45861-014
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 20, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
-
WARNINGS
- For external use only. Use only as directed. Avoid contact with eyes and mucous membranes.
- Do not cover with bandage.
- Do not use on wounds or damaged skin.
- Consult physician for children under 12.
- Do not use if you are allergic to Menthol
- Stop use and ask a doctor if conditions worsen, symptoms persist for more than 7 days or clear up and occur again within a few days
- Or rash, itching or excessive skin irritation occurs.
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- Adults and children 12 years and over apply to affected area: change patch 1 to 2 times daily
- Children under 12 years, consult physician before use
- How to apply:
- Clean and dry affected area
- Cut open pouch and remove patch
- Remove protective film and apply directly to area of pain
- Apply to affected area not more than 3 times daily
- Wash hands with soap after applying patch
- Reseal pouch containing unused patches
- OTHER INGREDIENTS
- MEDROX (MENTHOL, CAPSAICIN ) PATCH
-
INGREDIENTS AND APPEARANCE
MEDROX
menthol, capsaicin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50436-9991(NDC:45861-014) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.0375 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50436-9991-1 5 g in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/13/2012 Labeler - Unit Dose Services (831995316) Registrant - Unit Dose Services (831995316) Establishment Name Address ID/FEI Business Operations Unit Dose Services 831995316 REPACK(50436-9991)