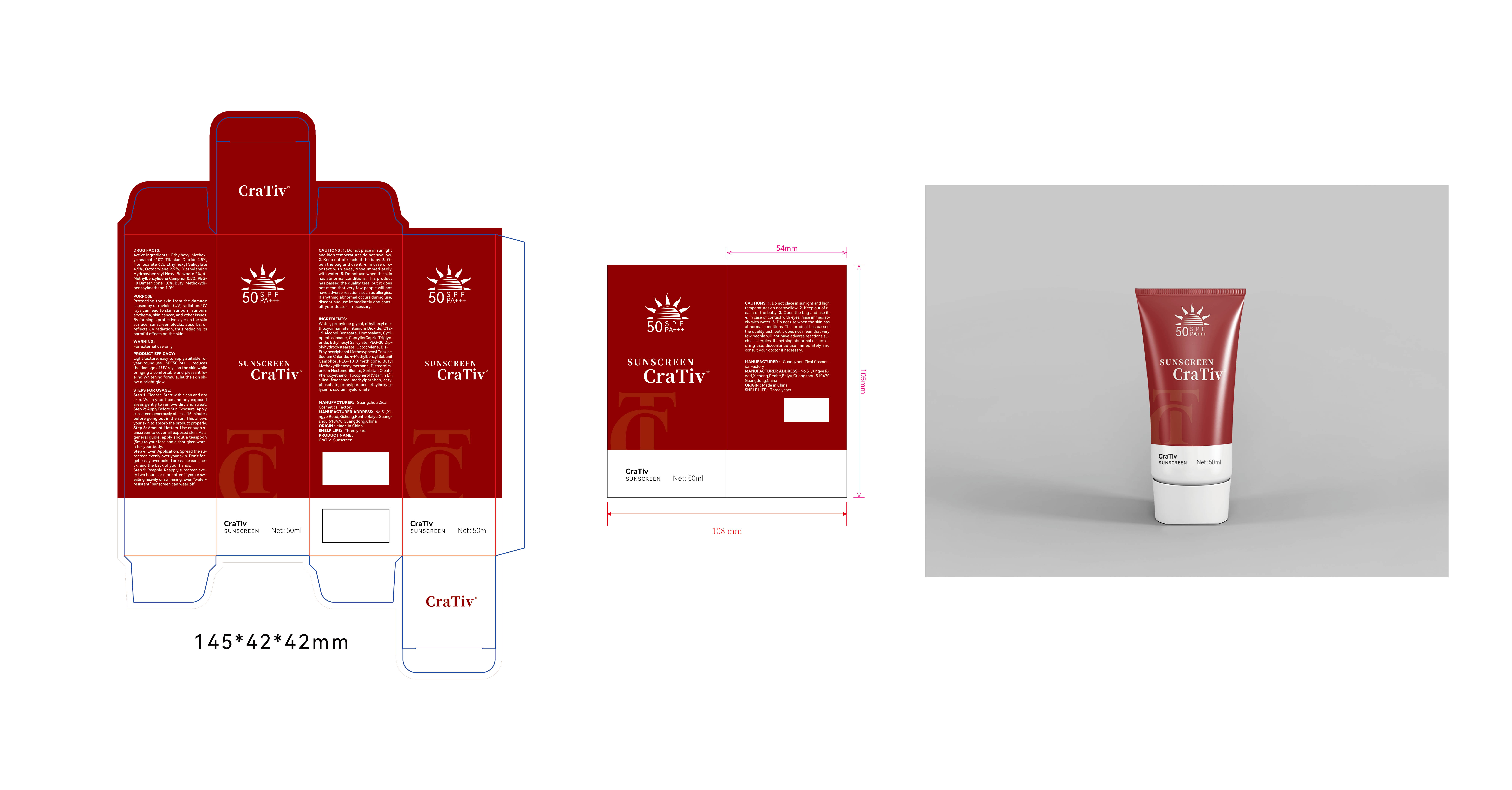
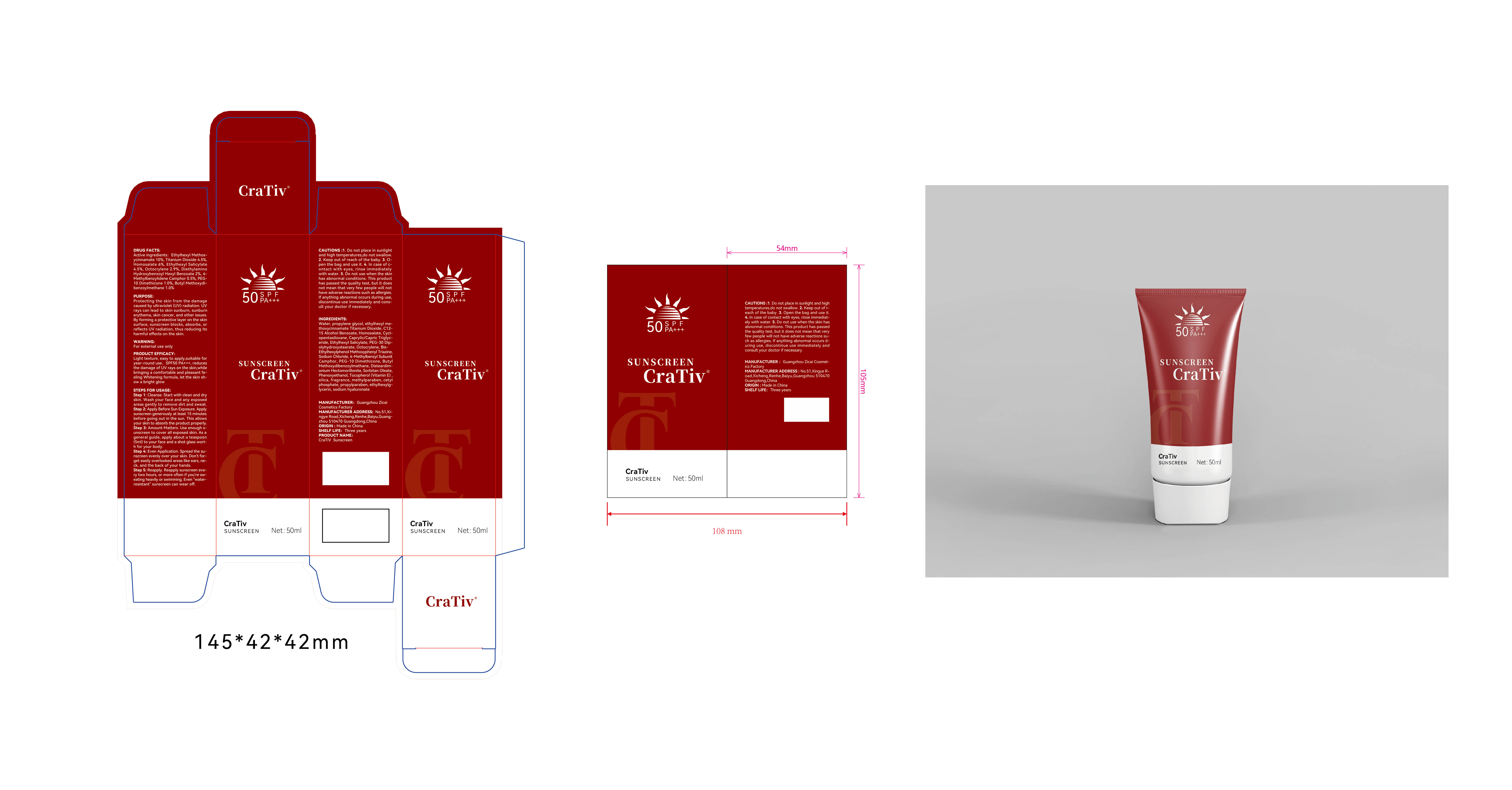
Label: CRATIV SUNSCREEN- ethylhexyl methoxycinnamate,titanium dioxide, homosalate, ethylhexyl, salicylate, octocrylene, bis-ethylhexyloxyphenolmethoxyphenyltriazine,4-methylbenzylidene camphor, dimethicone,butyl methoxydibenzoylmethane lotion
- NDC Code(s): 84301-001-01
- Packager: Foshan Xinhai Jiayu Import and Export Trade Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Step 1: Cleanse. Start with clean and dryskin. Wash your face and any exposedareas gently to remove dirt and sweat.
Step 2: Apply Before Sun Exposure. Applysunscreen generously at least 15 minutesbefore going out in the sun. This allowsyour skin to absorb the product properly.
Step 3: Amount Matters. Use enough s-unscreen to cover all exposed skin. As ageneral guide, apply about a teaspoon(5ml) to your face and a shot glass wort-h for your body.
Step 4: Even Application. Spread the su-nscreen evenly over your skin. Don't for-get easily overlooked areas like ears, ne-ck, and the back of your hands.
Step 5: Reapply. Reapply sunscreen eve-ry two hours, or more often if you're sw-eating heavily or swimming. Even water-resistant' sunscreen can wear off. - STORAGE AND HANDLING
-
INACTIVE INGREDIENT
water
propylene glycol
C12-15 Alcohol Benzoate
cyclopentasiloxane
Caprylic/capric triglyceride
PEG-30 Dipolyhydroxystearate
Sodium chloride
MONTMORILLONITE
Sorbitan oleate
Phenoxyethanol
.ALPHA.-TOCOPHEROL
SILICON DIOXIDE
Methylparaben
CETYL PHOSPHATE
Propylparaben
Ethylhexylglycerin
Sodium hyaluronate - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CRATIV SUNSCREEN
ethylhexyl methoxycinnamate,titanium dioxide, homosalate, ethylhexyl, salicylate, octocrylene, bis-ethylhexyloxyphenolmethoxyphenyltriazine,4-methylbenzylidene camphor, dimethicone,butyl methoxydibenzoylmethane lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84301-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.9 g in 100 mL BEMOTRIZINOL (UNII: PWZ1720CBH) (BEMOTRIZINOL - UNII:PWZ1720CBH) BEMOTRIZINOL 2 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 6 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.5 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL ENZACAMENE (UNII: 8I3XWY40L9) (ENZACAMENE - UNII:8I3XWY40L9) ENZACAMENE 0.5 g in 100 mL DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength MONTMORILLONITE (UNII: A585MN1H2L) CETYL PHOSPHATE (UNII: VT07D6X67O) SODIUM CHLORIDE (UNII: 451W47IQ8X) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PEG-30 DIPOLYHYDROXYSTEARATE (UNII: 9713Q0S7FO) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84301-001-01 50 mL in 1 TUBE; Type 0: Not a Combination Product 05/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/11/2024 Labeler - Foshan Xinhai Jiayu Import and Export Trade Co., Ltd (412478748) Establishment Name Address ID/FEI Business Operations Foshan Xinhai Jiayu Import and Export Trade Co., Ltd 412478748 manufacture(84301-001)