Label: STOOL COLLECTION KIT FOR GI MOLECULAR TEST- kit
- NHRIC Code(s): 70049-211-70
- NDC Code(s): 21749-363-76
- Packager: H&M MEDICAL COLLECTION KITS LLC
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated September 1, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
KIT Includes
- Lab Tissue Mailer, Overall Height 3-7/8 In., Overall Length 4-1/8 In., Zoro Number: G3670484, Mfr Number: 729/CP; Qty-1
- "Mini Ice Pack from an Ice pack Sqaure Mat, Length 16-1/2 In., Width 14-3/4 In., Height 14-3/4 In., Zoro #: G5830133 | Mfr #: 405SQ ( each matt has 60 pieces, which only one small piece is used)
- Mailing Carton, Capacity 72 cu. in., Color White, Inside Width 4 In., Inside Length 6 In., Inside Depth 3 In,Zoro#: G1072556 | Mfr#: 0101-1681010; Qty-1
- One 3ml transfer pipette; Transfer Pipet, 3.0mL, Small Bulb, Graduated to 1mL, 140mm, Bulb Draw - 2.2mL, globe scientific, item#137035; Qty-1
- One pair of medium latex free gloves
- Bag, Biohazard Specimen Transport, 6" x 9", Ziplock with Document Pouch, Globe scientific,item#4919; Qty-1
- Container, Fecal, 30mL, attached Screw Cap with Spoon, PP, Conical Bottom, Self-Standing, globe scientific, item#109120; Qty-1
- Sterile cotton tipped applicators, Dynarexcorporation, 2 X 6"inch, reorder no. 4305, lot: 25519; Qty-1
- Microtube, 1.5mL, Self-Standing, Attached Screw Cap for Color Insert, with O-Ring, STERILE, PP, globe scientific, item#111720; Qty-2
- Alcohol pads: PT# 5110 PT# # 5110- Prep Surgical Alcohol Pads Webcol Large by, Kendall Company Qty: 2 in each kit
- 1A Mini zip lock with a toilet cover seat: The Hospeco Health Gards half-fold toilet seat cover (part number:HG-1000) which is located inside a mini zip lock. Qty:1
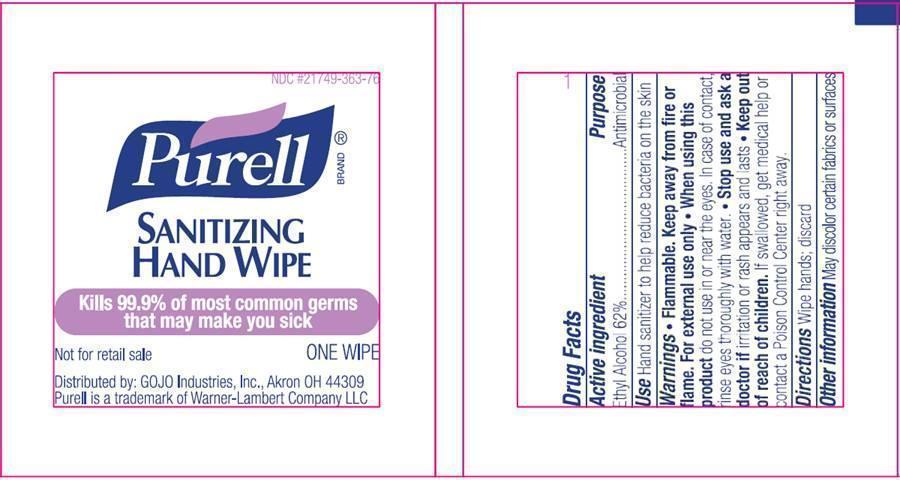
- PURELL Sanitizing Hand Wipes Individually Wrapped: Qty;1 in each kit
- Labels for patient identification
- Instruction sheet for collection the sample
- Requisition form for the clinicians
- Drug Facts
- Active ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- Other information
-
Inactive ingredients
Water (Aqua), Isopropyl Alcohol, Acetamidopropyl Trimonium Chloride, Aloe Barbadensis Leaf Juice, C12-15 Alkyl Benzoate, Cocamidopropyl PG-Dimonium Chloride Phosphate, Glycerin, Isopropyl Myristate, Propylene Glycol, Retinyl Palmitate, Tocopheryl Acetate, Zea Mays (Corn) Oil, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Fragrance (Parfum), Methylparaben
- Questions?
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
STOOL COLLECTION KIT FOR GI MOLECULAR TEST
container, specimen, non-sterile kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:70049-211 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:70049-211-70 1 in 1 KIT; Type 1: Convenience Kit of Co-Package Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 APPLICATOR 1.75 mL Part 1 of 1 PURELL SANITIZING HAND WIPES
ethyl alcohol clothProduct Information Item Code (Source) NDC:21749-363 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) ALOE VERA LEAF (UNII: ZY81Z83H0X) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CORN OIL (UNII: 8470G57WFM) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21749-363-76 1 in 1 PACKET 1 1.75 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/22/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device NNI 09/01/2015 Labeler - H&M MEDICAL COLLECTION KITS LLC (079931322) Establishment Name Address ID/FEI Business Operations H&M MEDICAL COLLECTION KITS LLC 079931322 repack Establishment Name Address ID/FEI Business Operations GOJO Industries, Inc 036424534 manufacture