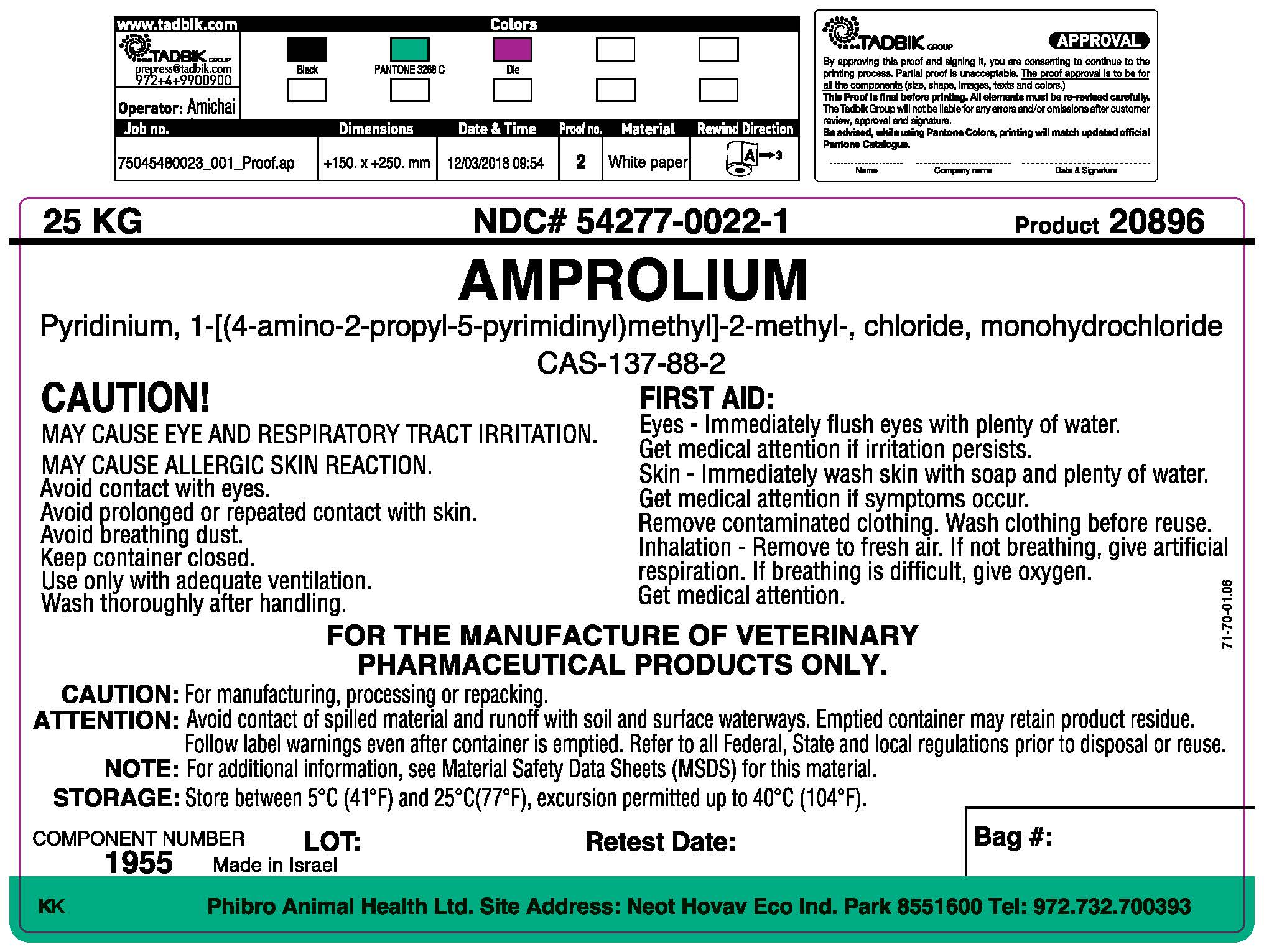
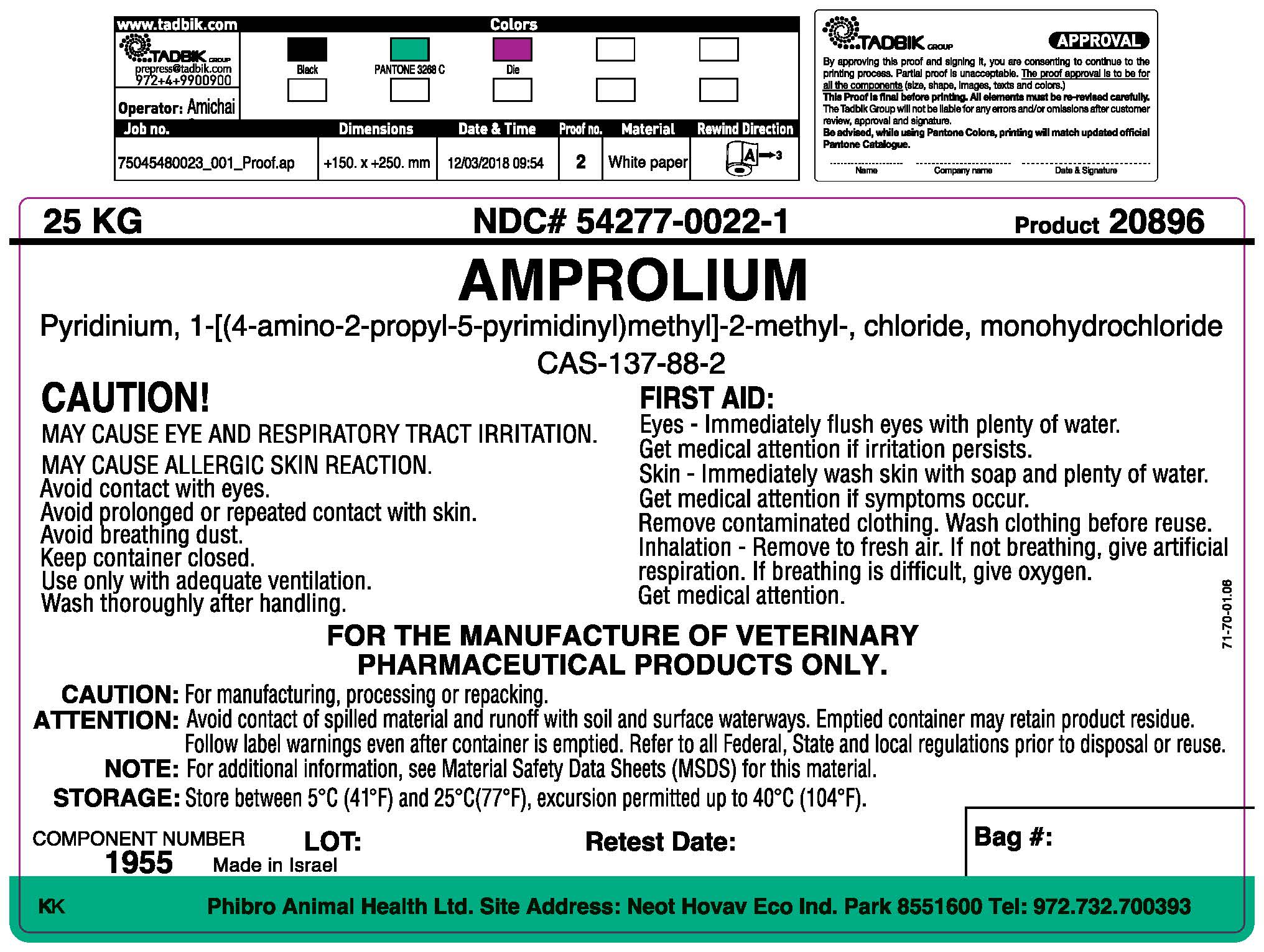
Label: AMPROLIUM powder
- NDC Code(s): 54277-0022-1
- Packager: Phibro Animal Health Ltd.
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: bulk ingredient
Drug Label Information
Updated May 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Package Label/Prinicipal Display Panel
- Package Label/Prinicipal Display Panel
-
FIRST AID:
Eye - Immediately flush eyes with plenty of water.
Get medical attention if irritation persists
Skin - Immediately wash skin with soap and plenty of water.
Get Medical attention if symptoms occur.
Remove contaminated clothing. Wash clothing before reuse.
Inhalation - Remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention.
-
FOR MANUFACTURING OF VETERINARY PHARMACEUTICAL PRODUCTS ONLY.
Caution: For manufacturing, processing or repacking. Attention: Avoid contact of spilled material and runoff with soil and surface waterways. Emptied container may retain product residue. Follow label warnings even after container is emptied. Refer to all Federal, State and local regulations prior to disposal or reuse. Note: For additional information, see Material Safety Data Sheets (MSDS) for this material.
Storage: Store between 5oC (41oF) and 25oC (77oF), excursions permitted up to 40oC (104oF).
COMPONENT NUMBER: 1955
Net Weight: 25 kg
- Made in Israel
- Label
-
INGREDIENTS AND APPEARANCE
AMPROLIUM
amprolium powderProduct Information Product Type Item Code (Source) NDC:54277-0022 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPROLIUM (UNII: 95CO6N199Q) (AMPROLIUM - UNII:95CO6N199Q) AMPROLIUM 1 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54277-0022-1 25 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Bulk ingredient 06/27/1990 Labeler - Phibro Animal Health Ltd. (600016711) Registrant - Phibro Animal Health (006989008)