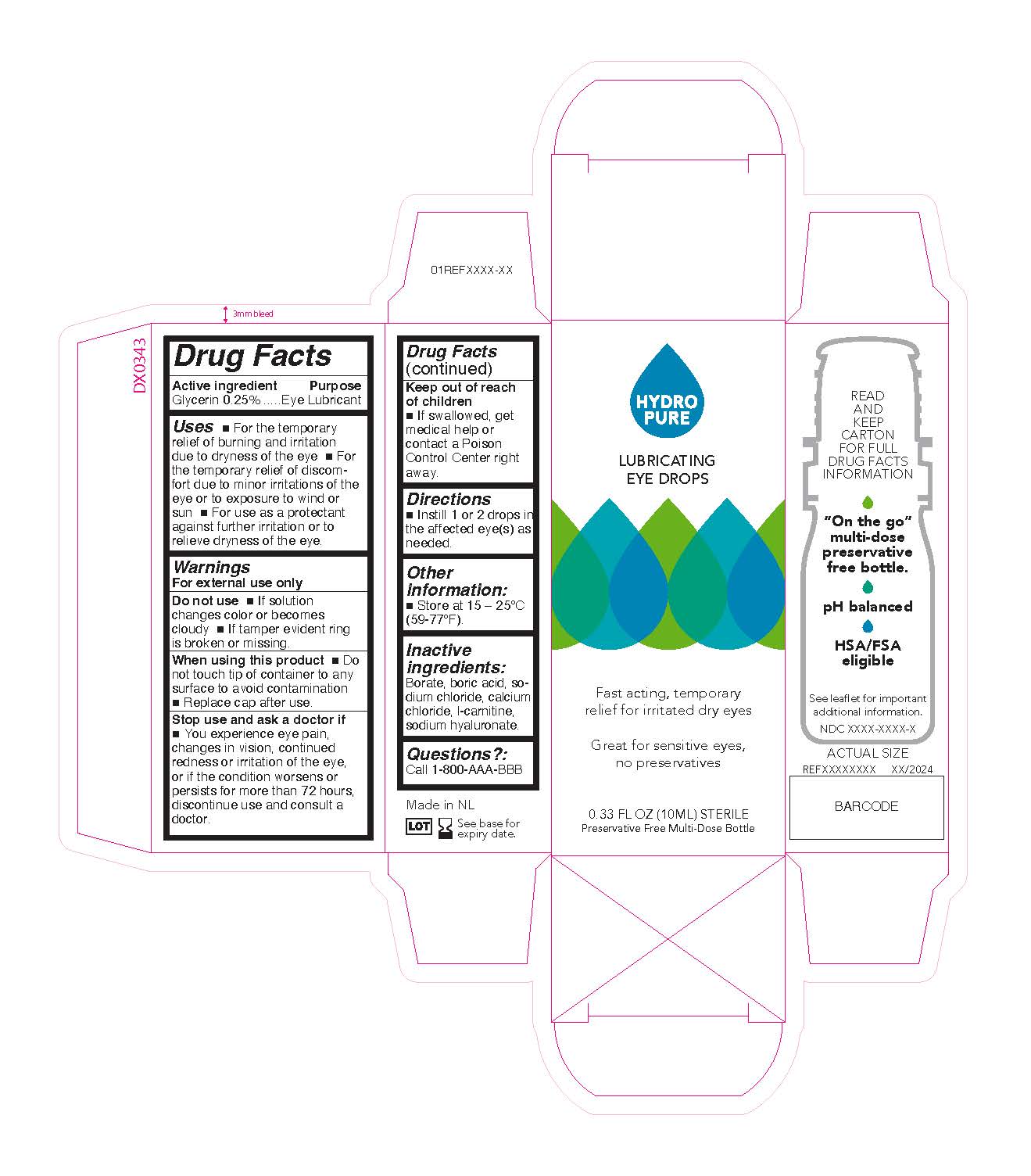
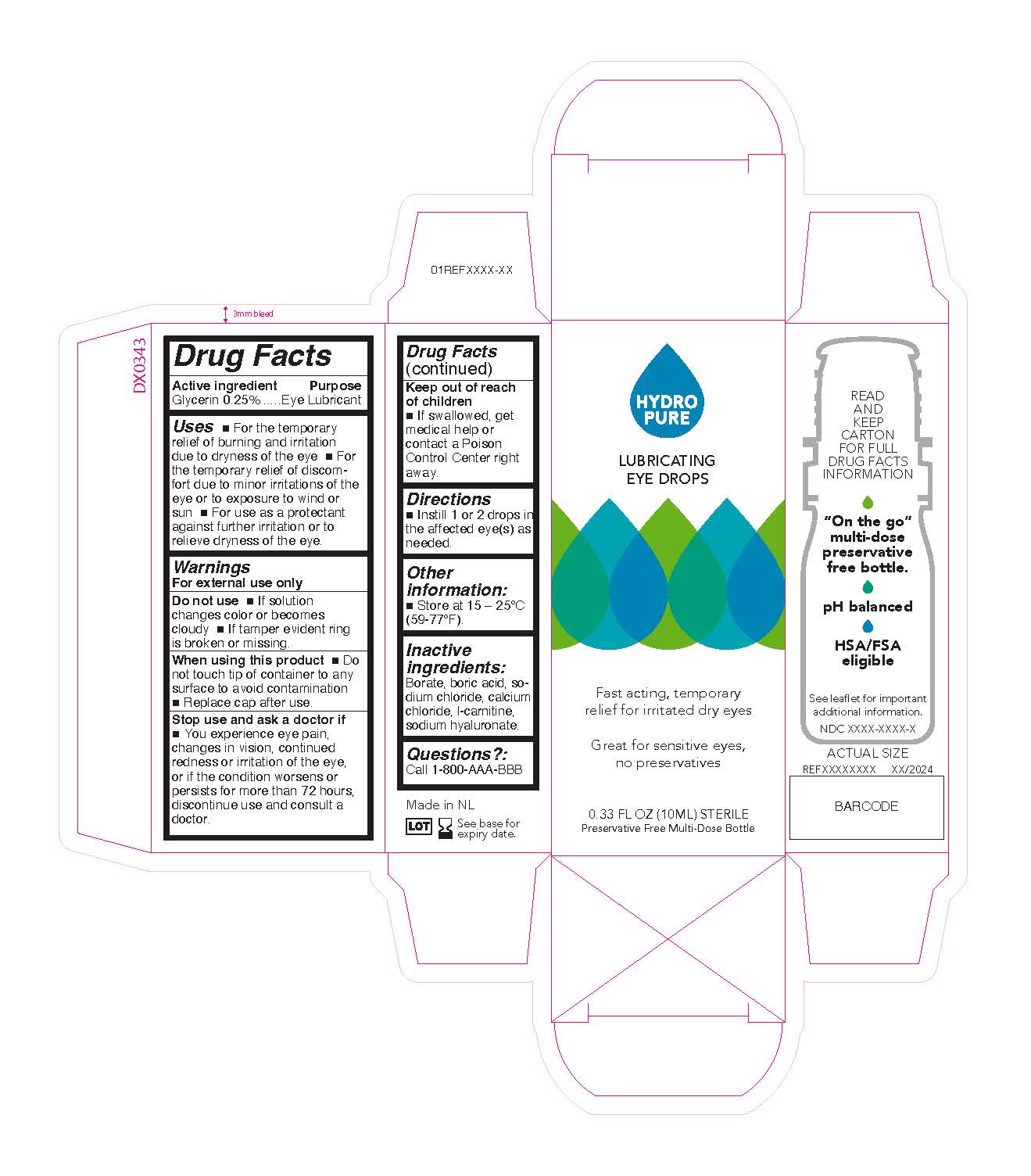
Label: 085 LUBRICATING EYE DROPS- lubricating eye drops solution/ drops
- NDC Code(s): 84227-851-10
- Packager: OTE North America
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
-
PURPOSE
I am not sure what is meant by purpose. There are the uses which is this section:
Uses
*For the temporary relief of burning and irritation due to dryness of the eye * For the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun * For use as a protectant against further irritation or to temporarily relieve dryness of the eye
Then in the active ingredients section it describes the purpose like this:
Active ingredient Purpose
Glycerin 0.25%................................... Eye Lubricant
- ACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
085 LUBRICATING EYE DROPS
lubricating eye drops solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84227-851 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOCARNITINE (UNII: 0G389FZZ9M) BORATE ION (UNII: 44OAE30D22) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84227-851-10 1 in 1 CARTON 05/15/2024 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 05/15/2024 Labeler - OTE North America (963905034)