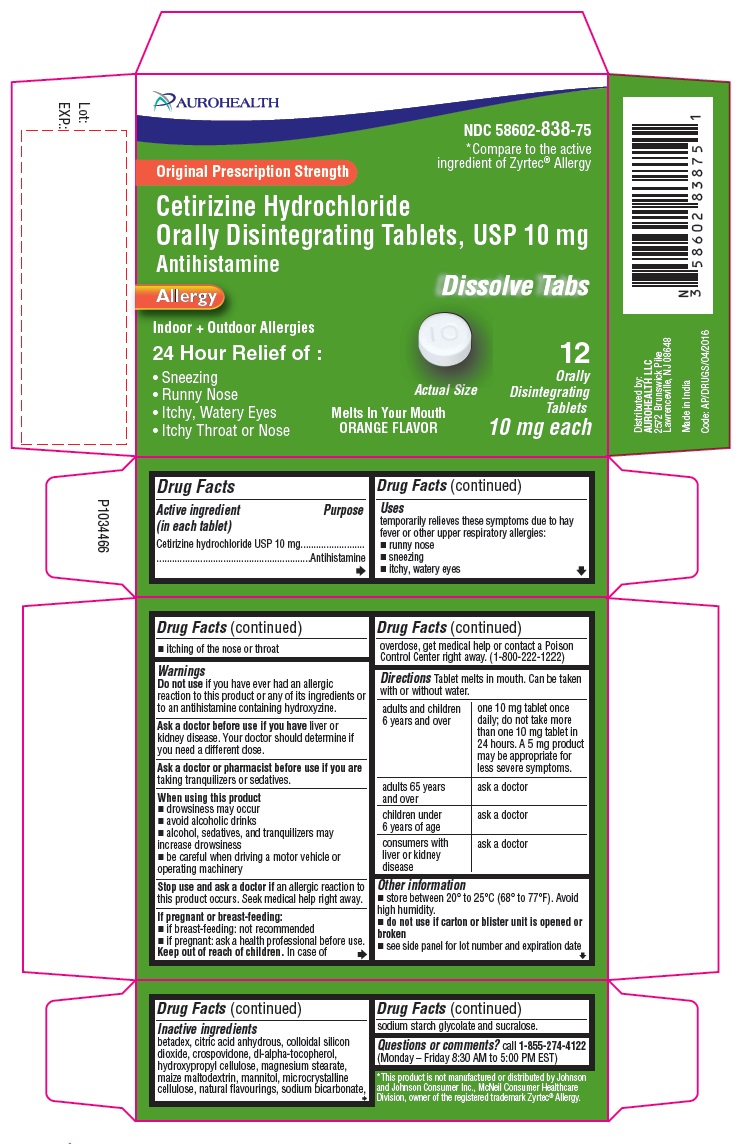
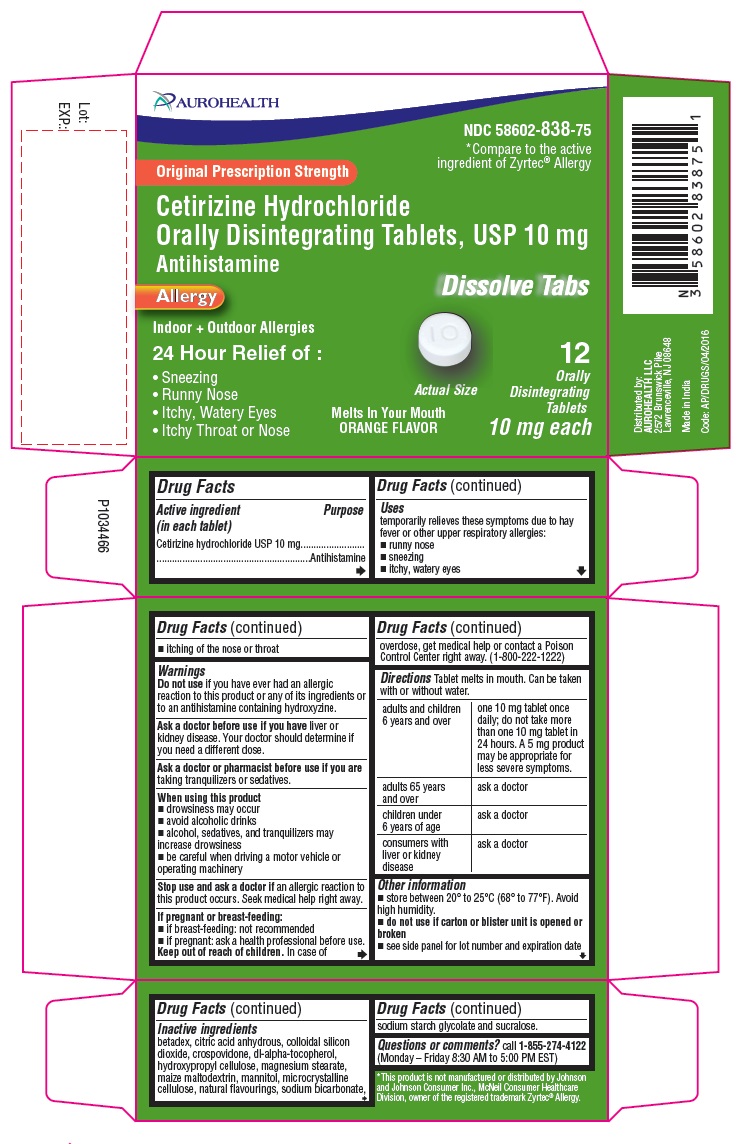
Label: CETIRIZINE HYDROCHLORIDE tablet, orally disintegrating
- NDC Code(s): 58602-838-14, 58602-838-75, 58602-838-76
- Packager: Aurohealth LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding:
- Keep out of reach of children.
-
Directions
Tablet melts in mouth. Can be taken with or without water.
adults and children 6 years and over
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
-
Inactive ingredients
betadex, citric acid anhydrous, colloidal silicon dioxide, crospovidone, dl-alpha-tocopherol, hydroxypropyl cellulose, magnesium stearate, maize maltodextrin, mannitol, microcrystalline cellulose, natural flavourings, sodium bicarbonate, sodium starch glycolate and sucralose.
Questions or comments?
call 1-855-274-4122 (Monday - Friday 8:30 AM to 5:00 PM EST)
Distributed by: AUROHEALTH LLC
2572 Brunswick Pike
Lawrenceville, NJ 08648Made in India
Code: AP/DRUGS/04/2016
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -10 mg (12 Orally Disintegrating Tablets) Blister Carton
AUROHEALTH
NDC 58602-838-75
*Compare to the active
ingredient of Zyrtec® Allergy
Original Prescription Strength
Cetirizine Hydrochloride
Orally Disintegrating Tablets, USP 10 mg
Antihistamine
Allergy
Dissolve Tabs
Indoor + Outdoor Allergies
24 Hour Relief of:- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
Melts In Your Mouth
ORANGE FLAVOR
Actual Size12 Orally
Disintegrating
Tablets
10 mg each
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58602-838 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength BETADEX (UNII: JV039JZZ3A) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (35 .MU.M) (UNII: 40UAA97IT9) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor ORANGE Imprint Code CE;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58602-838-75 2 in 1 CARTON 09/11/2020 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:58602-838-76 4 in 1 CARTON 09/11/2020 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:58602-838-14 11 in 1 CARTON 09/11/2020 3 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213557 09/11/2020 Labeler - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650918514 ANALYSIS(58602-838) , MANUFACTURE(58602-838)