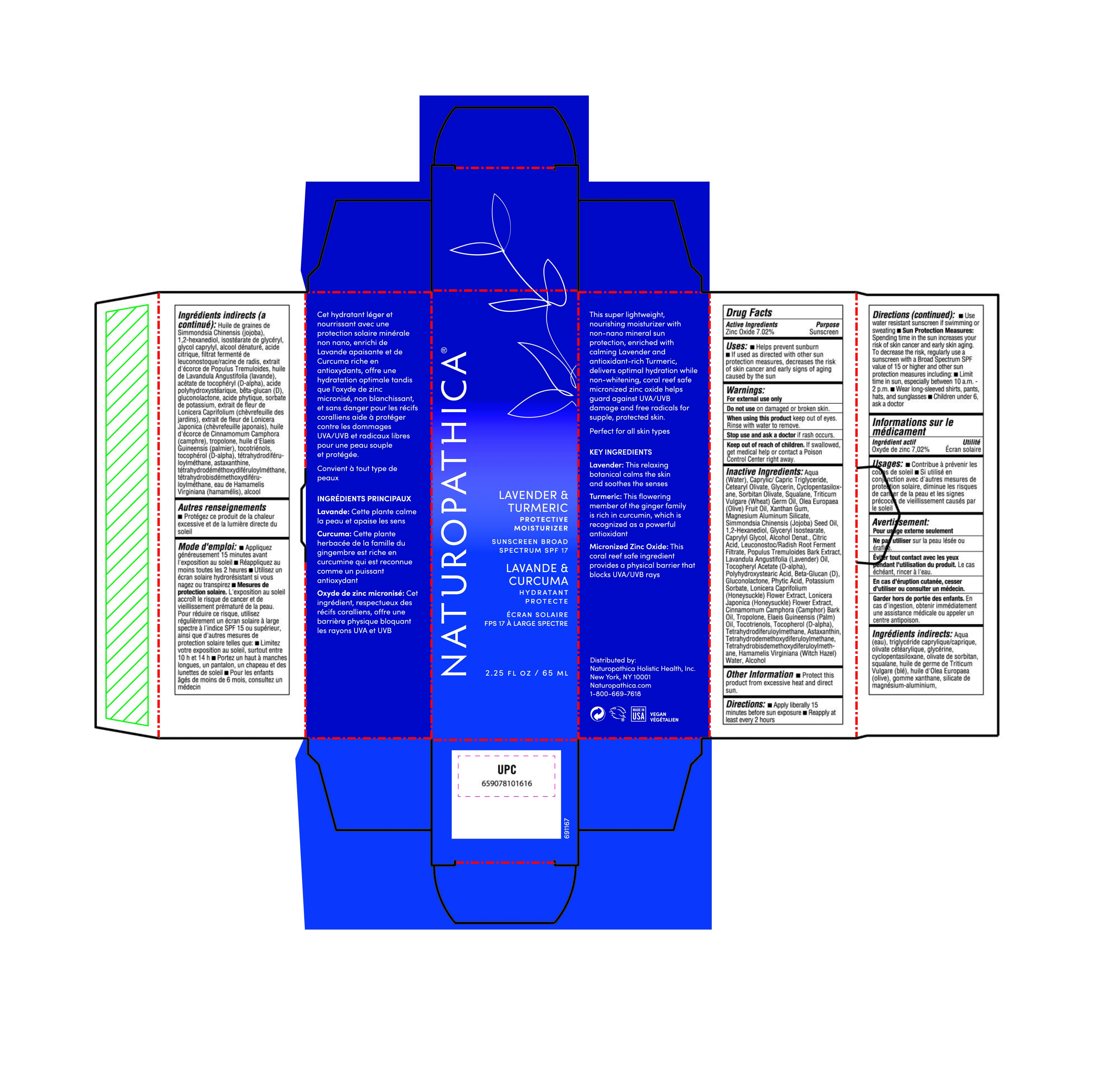
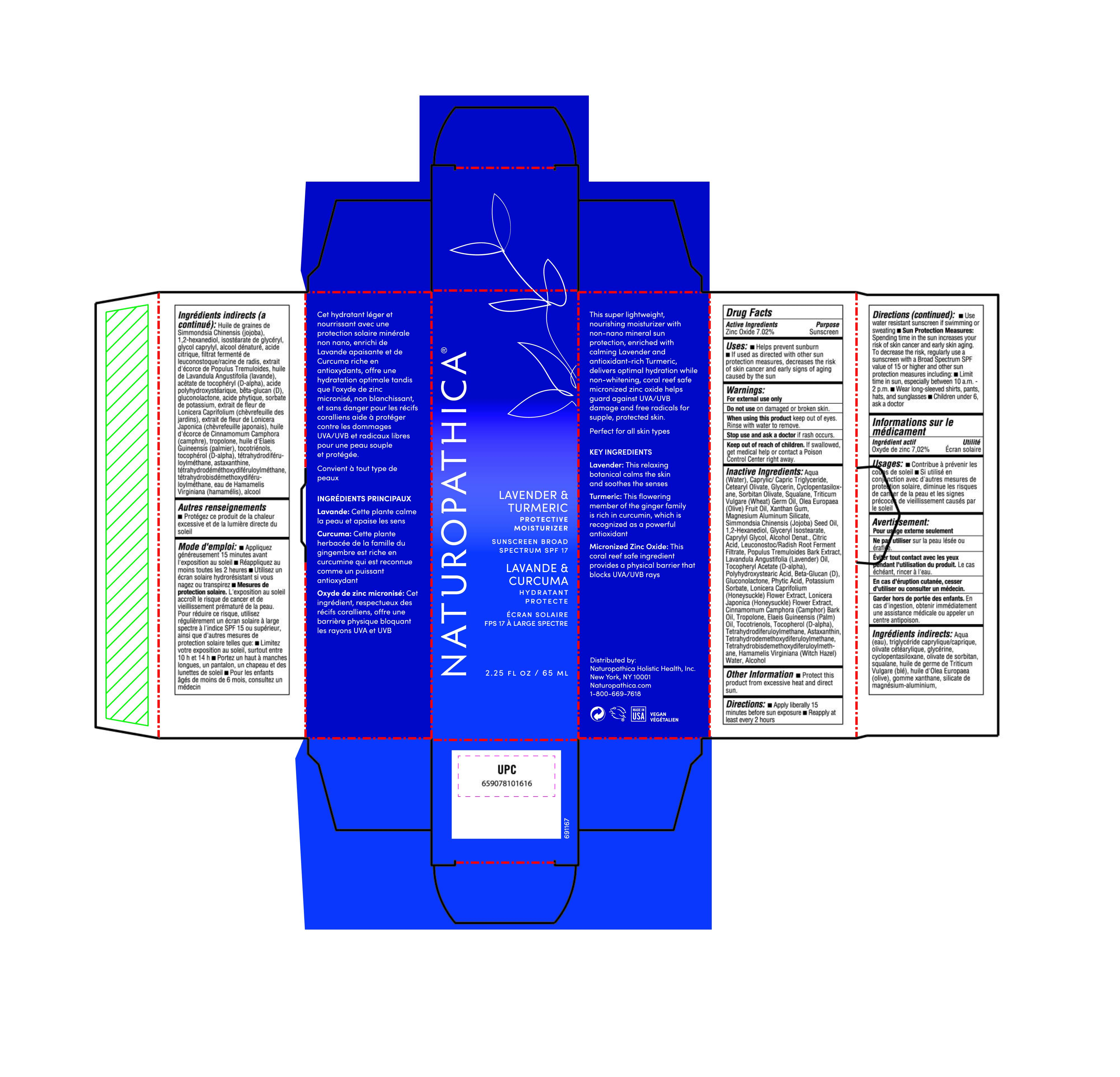
Label: LAVENDER PROTECTIVE MOISTURIZER SPF 17- zinc oxide lotion
- NDC Code(s): 64657-006-05
- Packager: Naturopathica Holistic Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Uses
• Helps prevent sunburn
• If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early signs of aging caused by the sun
- Warning
- KEEP OUT OF REACH OF CHILDREN
-
Inactive Ingredients
Aqua (Water), Caprylic/ Capric Triglyceride, Cetearyl Olivate, Glycerin, Cyclopentasiloxane, Sorbitan olivate, Squalane, Triticum Vulgare (Wheat) Germ Oil, Olea Europaea (Olive) Fruit Oil, Xanthan Gum, Magnesium Aluminum Silicate, Simmondsia Chinensis (Jojoba) Seed Oil, 12-Hexanediol, Glyceryl lsostearate, Caprylyl Glycol, Alcohol Denat., Citric Acid, Leuconostoc/Radish Root Ferment Filtrate, Populus Tremuloides Bark Extract, Lavandula Angustifolia (Lavender) Oil, Tocopheryl Acetate (D-alpha), Polyhydroxystearic Acid, Beta-Glucan (D), Gluconolactone, Phytic Acid, Potassium Sorbate, Lonicera Caprifolium (Honeysuckle) Flower Extract, Lonicera Japonica (Honeysuckle) Flower Extract, Cinnamomum Camphora (Camphor) Bark Oil, Tropolone, Elaeis Guineensis (Palm) oil, Tocotrienols, Tocopherol (D-alpha), Tetrahydrodiferuloylmethane, Astaxanthin, Tetrahydrodemethoxydiferuloylmethane, Tetrahydrobisdemethoxydiferuloylmethane, Hamamelis Virginiana (Witch Hazel) Water, Alcohol,
- Other information
-
Directions
• Apply liberally 15 minutes before sun exposure
• Reapply at least every 2 hours
• Use water resistant sunscreen if swimming or sweating
• Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• Limit lime in sun, especially between 10 a.m. - 2 p.m.
• Wear long-sleeved shirts, pants, hats, and sunglasses
• Children under 6, ask a doctor
- Lavender Protective Moisturizer SPF 17
-
INGREDIENTS AND APPEARANCE
LAVENDER PROTECTIVE MOISTURIZER SPF 17
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64657-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.02 g in 100 mL Inactive Ingredients Ingredient Name Strength TETRAHYDRODEMETHOXYDIFERULOYLMETHANE (UNII: 44D8X9U00T) FYTIC ACID (UNII: 7IGF0S7R8I) POPULUS TREMULOIDES BARK (UNII: 5543O0CEID) LAVENDER OIL (UNII: ZBP1YXW0H8) WATER (UNII: 059QF0KO0R) WHEAT GERM OIL (UNII: 14C97E680P) TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) XANTHAN GUM (UNII: TTV12P4NEE) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CETEARYL OLIVATE (UNII: 58B69Q84JO) SORBITAN OLIVATE (UNII: MDL271E3GR) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) SQUALANE (UNII: GW89575KF9) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) JOJOBA OIL (UNII: 724GKU717M) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) CAPRYLYL GLYCOL (UNII: 00YIU5438U) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) TOCOTRIENOLS (UNII: KP2MW85SSQ) .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) ASTAXANTHIN (UNII: 8XPW32PR7I) HAMAMELIS VIRGINIANA FLOWER WATER (UNII: 222MYC9QUV) GLUCONOLACTONE (UNII: WQ29KQ9POT) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) YEAST .BETA.-D-GLUCAN (UNII: 44FQ49X6UN) CAMPHOR OIL (UNII: 75IZZ8Y727) TETRAHYDROBISDEMETHOXYDIFERULOYLMETHANE (UNII: 973IBV8W7I) ALCOHOL (UNII: 3K9958V90M) PALM OIL (UNII: 5QUO05548Z) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CITRIC ACID ACETATE (UNII: DSO12WL7AU) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64657-006-05 65 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2017 Labeler - Naturopathica Holistic Health, Inc. (104302175) Establishment Name Address ID/FEI Business Operations Island Kinetics, Inc. d.b.a. CoValence Laboratories 959735002 manufacture(64657-006)