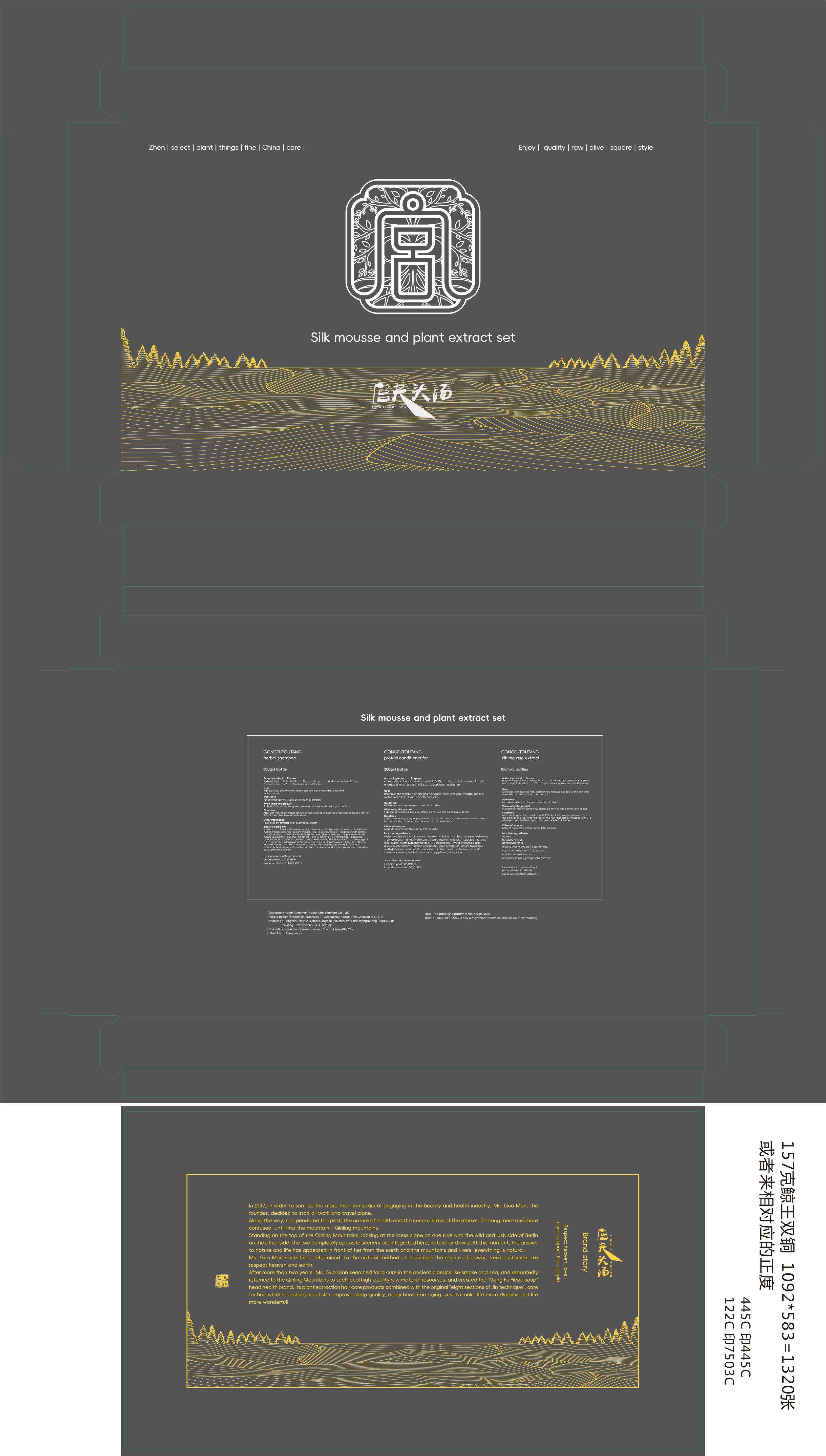
Label: GONGFUTOUYANG SILK MOUSSE EXTRACT liquid
GONGFUTOUYANG PROTEIN CONDITIONER FOR cream
GONGFUTOUYANG HERBAL SHAMPOO.- gongfutouyang herbal shampoo shampoo
- NDC Code(s): 84286-001-01, 84286-002-01, 84286-003-01
- Packager: Henan Chanman Health Management Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
-
Inactive ingredients
GONGFUTOUYANG herbal shampoo
water
cocamidopropyl betaine
sodium chloride
caprylyl/capryl glucoside
dimethicone
hydrogenated castor oil
sodium chloride
c12-18 alkyl alucoside
sodium laureth sulfate
cocamide mea
methyichloroisothiazolinone
methylisothiazolinone
magnesium chloride
magnesium nitrate
glycerin
aureth-23
c12-15 pareth-3,polyaminopropyl biguanide
amodimethicone,
steartrimonium chloride
isolaureth-6
dmdm hydantoin
butylene glycol
1,2-hexanediol
hydroxyacetophenone
caramel
guar hydroxypropyltrimonium chloride
methylparaben
allantoin
behenamidopropyl dimethylamine
panthenol
citric acid
aroma
polyquaternium-10
sodium acetate
sodium chloride
isopropyl alcohol
disodiumedta
piroctone olamineGONGFUTOUYANG protein conditioner for
water
cetearyl alcohol
ceteartrimonium chloride
alcohol
cyclopentasiloxanedimethicone
dimethicone
amodimethicone
steartrimonium chloride
isolaureth-6
buty-lene glycol
hydroxyacetophenone
1,2-hexanediol
hydroxyethylcellulose
disodium phosphate
sodium phosphate
polysorbate 60
dmdm hydantoin
methylparaben
citric acid
squalane
ci 19140
sodium chloride
ci 15985
camellia japonica seed oil
macrocystis pyrifera (kelp) proteinGONGFUTOUYANG silk mousse extract
water
butylene glycol
phenoxyethanol
glycine max (soybean) seed extract
capsicum frutescens fruit extract
eclipta prostrata extract
rehmanniae radix praeparata extract - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GONGFUTOUYANG SILK MOUSSE EXTRACT
gongfutouyang silk mousse extract liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84286-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANGELICA SINENSIS ROOT (UNII: B66F4574UG) (ANGELICA SINENSIS ROOT - UNII:B66F4574UG) ANGELICA SINENSIS ROOT 0.5 g in 100 mL PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) (PLATYCLADUS ORIENTALIS LEAF - UNII:32E5V7G32B) PLATYCLADUS ORIENTALIS LEAF 0.7 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SOYBEAN (UNII: L7HT8F1ZOD) ECLIPTA PROSTRATA LEAF (UNII: H86R96580E) TABASCO PEPPER (UNII: J1M3NA843L) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) REHMANNIA GLUTINOSA ROOT (UNII: 1BEM3U6LQQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84286-003-01 3 in 1 BOX 05/06/2024 1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/06/2024 GONGFUTOUYANG PROTEIN CONDITIONER FOR
gongfutouyang protein conditioner for creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84286-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANGELICA SINENSIS ROOT (UNII: B66F4574UG) (ANGELICA SINENSIS ROOT - UNII:B66F4574UG) ANGELICA SINENSIS ROOT 0.2 g in 100 g JOJOBA OIL (UNII: 724GKU717M) (JOJOBA OIL - UNII:724GKU717M) JOJOBA OIL 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) DMDM HYDANTOIN (UNII: BYR0546TOW) METHYLPARABEN (UNII: A2I8C7HI9T) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) CAMELLIA JAPONICA SEED OIL (UNII: U37N0S910T) MACROCYSTIS PYRIFERA (UNII: K31S3OG5C4) CETEARTRIMONIUM CHLORIDE (UNII: 34L9KA56IO) POLYSORBATE 60 (UNII: CAL22UVI4M) SODIUM CHLORIDE (UNII: 451W47IQ8X) AMODIMETHICONE (1300 CST) (UNII: 3V7U636DWN) TRIMETHYL OCTADECYL AMMONIUM CHLORIDE (UNII: CZ70647U92) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM PHOSPHATE (UNII: SE337SVY37) ACONITIC ACID (UNII: 93371T1BXP) SQUALANE (UNII: GW89575KF9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ALCOHOL (UNII: 3K9958V90M) CYCLOMETHICONE 7 (UNII: KCK5L8VU47) ISOLAURETH-6 (UNII: 0URO31WRVV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84286-002-01 300 g in 1 BOTTLE; Type 0: Not a Combination Product 05/06/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/06/2024 GONGFUTOUYANG HERBAL SHAMPOO.
gongfutouyang herbal shampoo shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84286-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAURETH-2 SULFATE (UNII: 27U3CUA29X) (LAURETH-23 - UNII:N72LMW566G) LAURETH-2 SULFATE 12.6 g in 100 g COCO DIETHANOLAMIDE (UNII: 92005F972D) (COCO DIETHANOLAMIDE - UNII:92005F972D) COCO DIETHANOLAMIDE 1.7 g in 100 g Inactive Ingredients Ingredient Name Strength C12-15 PARETH-3 (UNII: 459EF9MP3Y) POLYAMINOPROPYL BIGUANIDE (UNII: DT9D8Z79ET) SURFOMER (UNII: 95S6LH9UEV) WATER (UNII: 059QF0KO0R) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) MAGNESIUM NITRATE (UNII: 77CBG3UN78) AMODIMETHICONE (1300 CST) (UNII: 3V7U636DWN) TRIMETHYL OCTADECYL AMMONIUM CHLORIDE (UNII: CZ70647U92) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) PANTHENOL (UNII: WV9CM0O67Z) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) ISOLAURETH-6 (UNII: 0URO31WRVV) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CARAMEL (UNII: T9D99G2B1R) METHYLPARABEN (UNII: A2I8C7HI9T) ALLANTOIN (UNII: 344S277G0Z) BEHENAMIDOPROPYL DIMETHYLAMINE (UNII: X4O854526J) POLYQUATERNIUM-10 (1000 MPA.S AT 2%) (UNII: GMR4PEN8PK) SODIUM ACETATE (UNII: 4550K0SC9B) EDETATE DISODIUM (UNII: 7FLD91C86K) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM CHLORIDE (UNII: 451W47IQ8X) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) LAURETH-23 (UNII: N72LMW566G) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCO MONOETHANOLAMIDE (UNII: C80684146D) DMDM HYDANTOIN (UNII: BYR0546TOW) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) ACONITIC ACID (UNII: 93371T1BXP) ISOPROPYL ALCOHOL (UNII: ND2M416302) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84286-001-01 300 g in 1 BOTTLE; Type 0: Not a Combination Product 05/06/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/06/2024 Labeler - Henan Chanman Health Management Co., Ltd (707653765) Establishment Name Address ID/FEI Business Operations Guangzhou NOLAN Fine Chemical Co. LTD 553578577 manufacture(84286-001, 84286-002, 84286-003)