Label: ACNIL- acnil clindamycin hydrochloride and metronidazole liniment liniment
- NDC Code(s): 84277-5647-1
- Packager: Jiangsu Chenpai Bond Pharmaceutical Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION For external use only. Drip 1~2 drops of Acnil to affected area, or dip some Acnil in cotton bud and apply to the affected area. It should be applied three times daily. Two weeks’ treatment constitutes a single therapeutic course. Repeat another therapeutic course if it is necessary.
- WARNINGS
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
PHARMACOLOGY AND TOXICOLOGY Clindamycin Hydrochloride is a bacteriostatic antibiotic, which is 4 to 8 times more potent than Lincomycin. It has potent antibacterial activity to aerobic gram-positive bacterium, such as Staphylococcus, Streptococcus, Bacillus diphtheriae and Bacillus anthracis. It also has good antibacterial activity to anaerobic gram-negative bacterium. Bacteroides (including bacteroides fragilis) and Clostridium are morbidly susceptible to it. It binds exclusively to the 50'S subunit of sensitive bacterial ribosome and suppresses protein synthesis. Clindamycin is a bacteriostat, but can kill high sensitive bacterium at high concentration. Metronidazole is an anti-anaerobic bacterial, anti-trichomonal and anti-amebic agent. Combination of Clindamycin Hydrochloride and Metronidazole can kill Propionibacterium acnes, Pityrosporum ovale, and some other sensitive bacterium and vermiform mite, and can inhibit them from growing. As a result, it can reduce the creation of lipase, and prevent triacylglycerol from hydrolyzing into free fatty acid.
INDICATIONS It is indicated for the treatment of acne (especially for the acne pustulosa, cystic acne), tympanitis, nasosinusitis, folliculitis, and other bacterial infection on skin. It is also indicated for the supportive treatment of seborrheic dermatitis and brandy nose.
- KEEP OUT OF REACH OF CHILDREN
-
PURPOSE
DESCRIPTION Acolorless or slightly yellow clear liquid; with an odour of alcohol.
PHARMACOLOGY AND TOXICOLOGY Clindamycin Hydrochloride is a bacteriostatic antibiotic, which is 4 to 8 times more potent than Lincomycin. It has potent antibacterial activity to aerobic gram-positive bacterium, such as Staphylococcus, Streptococcus, Bacillus diphtheriae and Bacillus anthracis. It also has good antibacterial activity to anaerobic gram-negative bacterium. Bacteroides (including bacteroides fragilis) and Clostridium are morbidly susceptible to it. It binds exclusively to the 50'S subunit of sensitive bacterial ribosome and suppresses protein synthesis. Clindamycin is a bacteriostat, but can kill high sensitive bacterium at high concentration. Metronidazole is an anti-anaerobic bacterial, anti-trichomonal and anti-amebic agent. Combination of Clindamycin Hydrochloride and Metronidazole can kill Propionibacterium acnes, Pityrosporum ovale, and some other sensitive bacterium and vermiform mite, and can inhibit them from growing. As a result, it can reduce the creation of lipase, and prevent triacylglycerol from hydrolyzing into free fatty acid.
INDICATIONS It is indicated for the treatment of acne (especially for the acne pustulosa, cystic acne), tympanitis, nasosinusitis, folliculitis, and other bacterial infection on skin. It is also indicated for the supportive treatment of seborrheic dermatitis and brandy nose.
Drug Interactions: There is antagonism between ACNIL and erythromycin, so the combination of ACNIL and erythromycin is forbidden.
- ACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNIL
acnil clindamycin hydrochloride and metronidazole liniment linimentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84277-5647 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 8 mg in 30 mL CLINDAMYCIN HYDROCHLORIDE (UNII: T20OQ1YN1W) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 10 mg in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2-(ETHYLTHIO)ETHANOL (UNII: 6091RQ1378) GLYCEROL FORMAL (UNII: 3L7GR2604E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84277-5647-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2024 Labeler - Jiangsu Chenpai Bond Pharmaceutical Co.,Ltd. (561021529) Registrant - Jiangsu Chenpai Bond Pharmaceutical Co.,Ltd. (561021529) Establishment Name Address ID/FEI Business Operations Jiangsu Chenpai Bond Pharmaceutical Co.,Ltd. 561021529 manufacture(84277-5647)

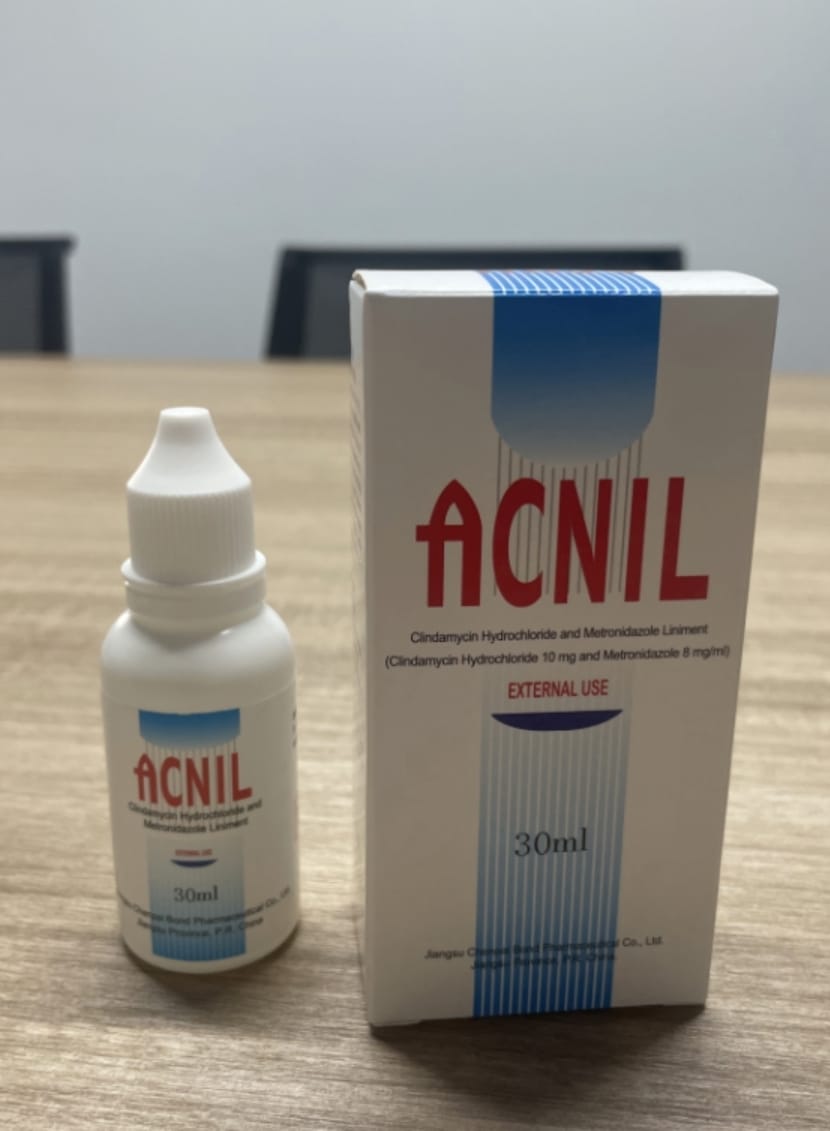

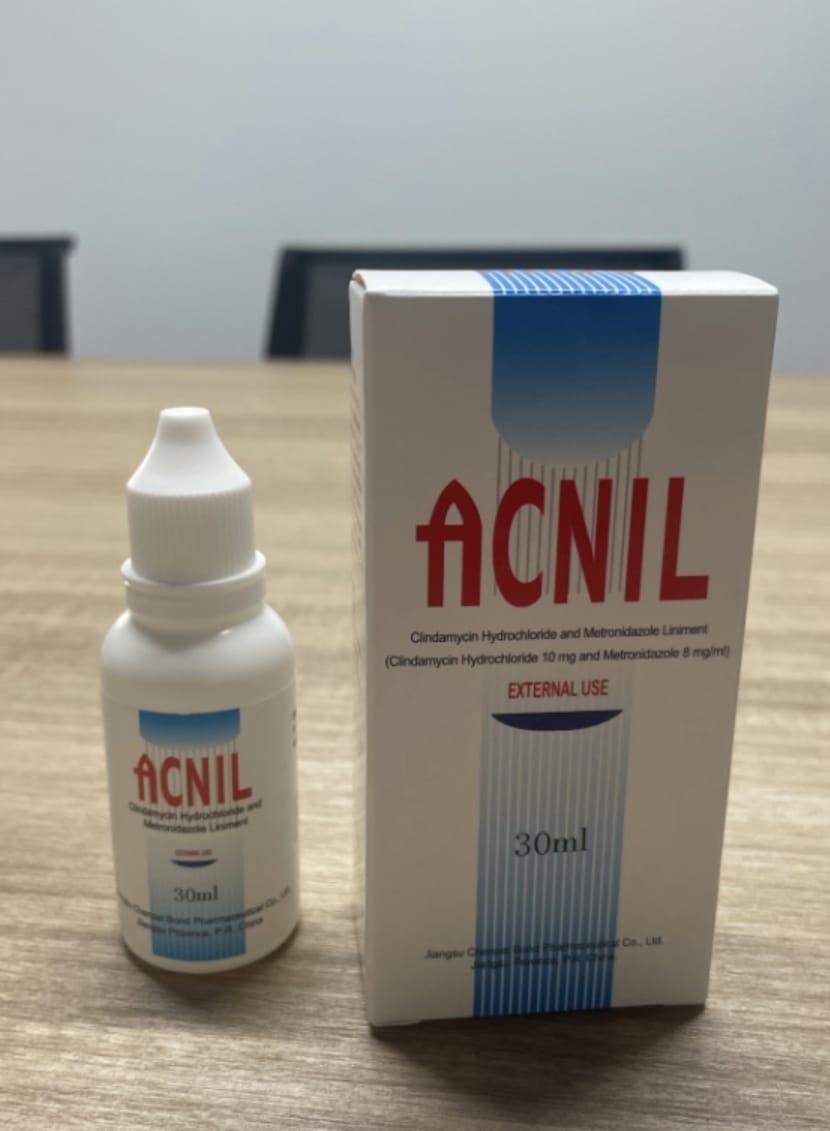
 Content of Labeling
Content of Labeling