Label: STREAM2SEA BROAD SPECTRUM 30- titanium dioxide lotion
STREAM2SEA BROAD SPECTRUM 30 - TINTED- titanium dioxide lotion
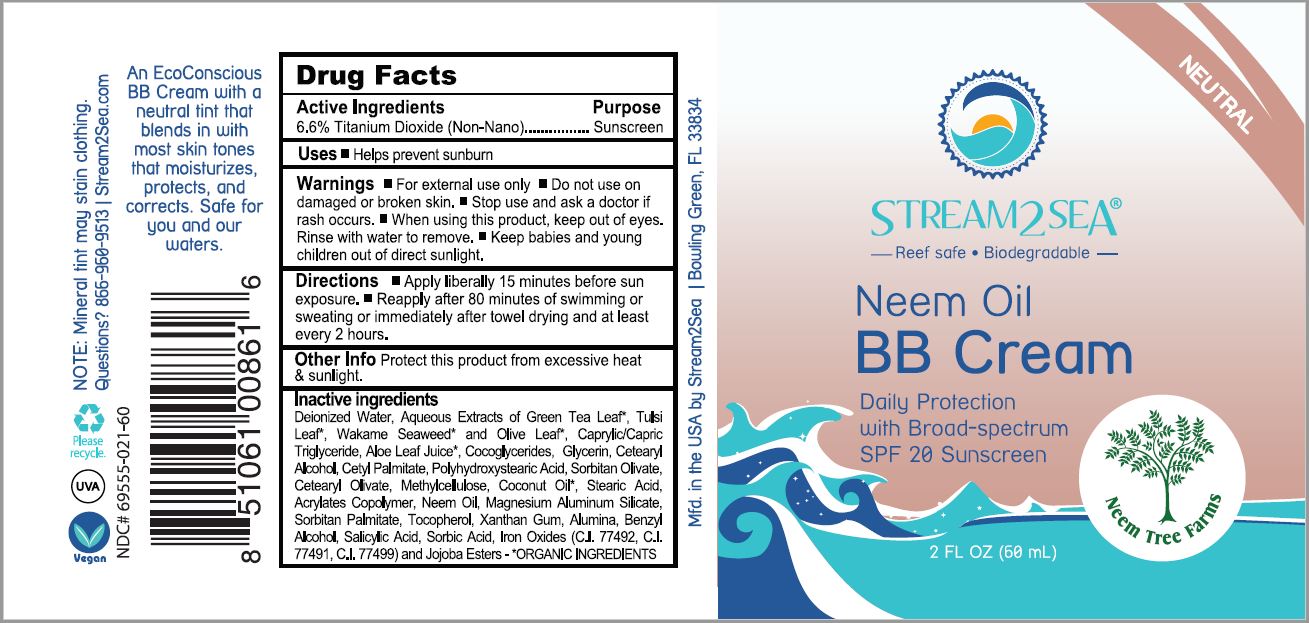
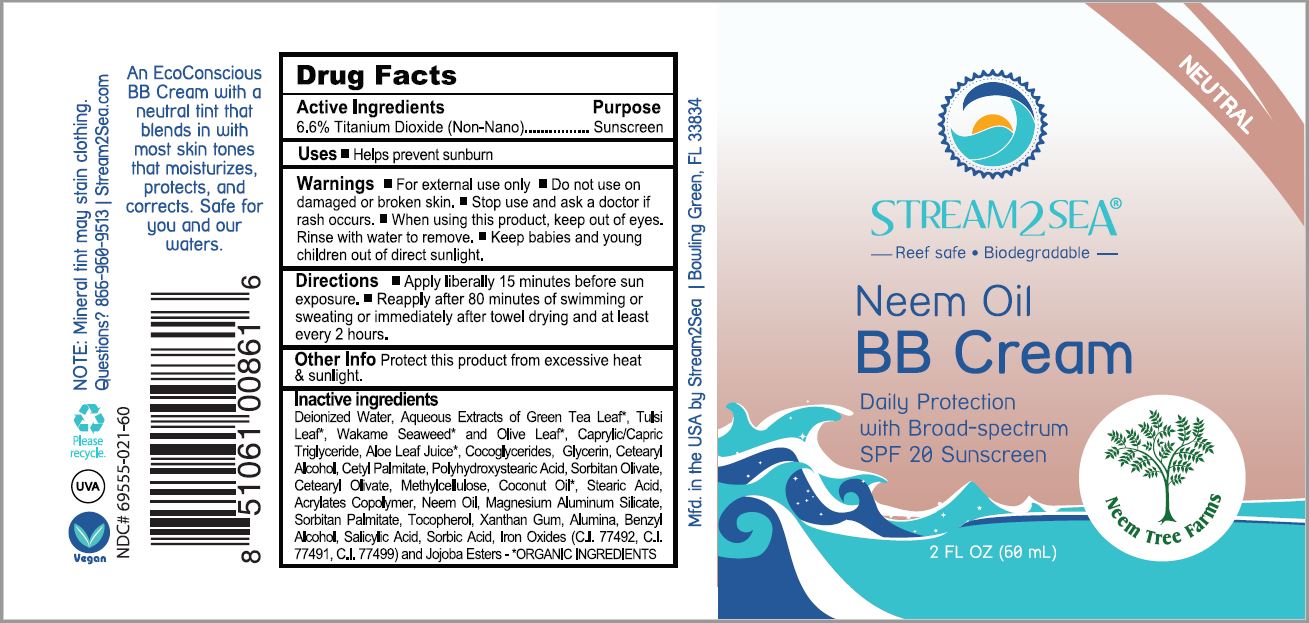
STREAM2SEA BROAD SPECTRUM 20- titanium dioxide lotion
STREAM2SEA BROAD SPECTRUM 20 - TINTED- titanium dioxide lotion
-
NDC Code(s):
69555-020-01,
69555-020-03,
69555-020-32,
69555-021-30, view more69555-021-32, 69555-021-60, 69555-021-90, 69555-030-01, 69555-030-03, 69555-030-30, 69555-030-32, 69555-030-33, 69555-031-30, 69555-031-32, 69555-031-90, 69555-031-91
- Packager: Stream2Sea, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Directions
- Warnings
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
TINTED SPF 30
Deionized Water, Aqueous Extracts of Camellia Sinensis (Green Tea) Leaf*, Ociimum Tenuiflorum (Tulsi) Leaf*, Alara Ssculenta (Wakame Seaweed)* and Olea Europa (Olive) Leaf*, Caprylic Triglyceride, Aloe Barbadensis (Aloe) Leaf Juice*, Cocoglycerides, Glycerin, Cetearyl Alcohol, Cetyl Palmitate, Polyhydroxystearic Acid, Sorbitan Olivate, Cetearyl Olivate, Methylcellulose, Cocos Nucifera (Coconut) Oil*, Stearic Acid, Acrylates Copolymer, Magnesium Aluminum Silicate, Sorbitan Palmitate, Tocopherol, Xanthan Gum, Alumina Benzyl Alcohol, Salicylic Acid, Sorbic Acid, Iron Oxides (CI 77492, CI 77499) and Jojoba Ester
*ORGANIC INGREDIENTS
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Stream2Sea Broad Spectrum Sport Face & Body SPF 20 1oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 20 3oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 20 32oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 20-TINTED 1oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 20-TINTED 2oz
Stream2Sea Broad Spectrum Sport Face & Body SPF 20-TINTED 3oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 20-TINTED 32oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 30 1oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 30 3oz

Stream2Sea Water Spot SPF 30 -3 FL OZ (90 ML)

Stream2Sea Broad Spectrum Sport Face & Body SPF 30 32oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 30-TINTED 1oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 30-TINTED 3oz

Stream2Sea Broad Spectrum Sport Face & Body SPF 30-TINTED 32oz

Happy Ocean Water Sport SPF 30 (75 mL)

Happy Ocean Water Sport SPF 30 Tinted (75 mL)

-
INGREDIENTS AND APPEARANCE
STREAM2SEA BROAD SPECTRUM 30
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69555-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 g in 88 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HOLY BASIL LEAF (UNII: SCJ765569P) ALARIA ESCULENTA (UNII: EJ9JK8J58D) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCO-GLYCERIDES (UNII: ISE9I7DNUG) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SORBITAN OLIVATE (UNII: MDL271E3GR) CETEARYL OLIVATE (UNII: 58B69Q84JO) METHYLCELLULOSE, UNSPECIFIED (UNII: Z944H5SN0H) COCONUT OIL (UNII: Q9L0O73W7L) STEARIC ACID (UNII: 4ELV7Z65AP) LUVISET 360 (UNII: 05BG6GY6YK) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) ALUMINUM OXIDE (UNII: LMI26O6933) BENZYL ALCOHOL (UNII: LKG8494WBH) SALICYLIC ACID (UNII: O414PZ4LPZ) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69555-030-01 30 mL in 1 TUBE; Type 0: Not a Combination Product 07/01/2015 2 NDC:69555-030-03 90 mL in 1 TUBE; Type 0: Not a Combination Product 07/01/2015 3 NDC:69555-030-30 90 mL in 1 TUBE; Type 0: Not a Combination Product 06/03/2023 4 NDC:69555-030-32 946 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 5 NDC:69555-030-33 75 mL in 1 TUBE; Type 0: Not a Combination Product 12/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/01/2015 STREAM2SEA BROAD SPECTRUM 30 - TINTED
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69555-031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 g in 88 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HOLY BASIL LEAF (UNII: SCJ765569P) ALARIA ESCULENTA (UNII: EJ9JK8J58D) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCO-GLYCERIDES (UNII: ISE9I7DNUG) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SORBITAN OLIVATE (UNII: MDL271E3GR) CETEARYL OLIVATE (UNII: 58B69Q84JO) METHYLCELLULOSE, UNSPECIFIED (UNII: Z944H5SN0H) COCONUT OIL (UNII: Q9L0O73W7L) STEARIC ACID (UNII: 4ELV7Z65AP) LUVISET 360 (UNII: 05BG6GY6YK) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) ALUMINUM OXIDE (UNII: LMI26O6933) BENZYL ALCOHOL (UNII: LKG8494WBH) SALICYLIC ACID (UNII: O414PZ4LPZ) SORBIC ACID (UNII: X045WJ989B) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69555-031-30 30 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 2 NDC:69555-031-90 90 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 3 NDC:69555-031-32 946 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 4 NDC:69555-031-91 75 mL in 1 TUBE; Type 0: Not a Combination Product 12/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/13/2021 STREAM2SEA BROAD SPECTRUM 20
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69555-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 g in 66 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HOLY BASIL LEAF (UNII: SCJ765569P) ALARIA ESCULENTA (UNII: EJ9JK8J58D) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCO-GLYCERIDES (UNII: ISE9I7DNUG) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SORBITAN OLIVATE (UNII: MDL271E3GR) CETEARYL OLIVATE (UNII: 58B69Q84JO) METHYLCELLULOSE, UNSPECIFIED (UNII: Z944H5SN0H) COCONUT OIL (UNII: Q9L0O73W7L) STEARIC ACID (UNII: 4ELV7Z65AP) LUVISET 360 (UNII: 05BG6GY6YK) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) ALUMINUM OXIDE (UNII: LMI26O6933) BENZYL ALCOHOL (UNII: LKG8494WBH) SALICYLIC ACID (UNII: O414PZ4LPZ) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69555-020-01 30 mL in 1 TUBE; Type 0: Not a Combination Product 07/01/2015 2 NDC:69555-020-03 90 mL in 1 TUBE; Type 0: Not a Combination Product 07/01/2015 3 NDC:69555-020-32 946 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/01/2015 STREAM2SEA BROAD SPECTRUM 20 - TINTED
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69555-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 g in 66 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HOLY BASIL LEAF (UNII: SCJ765569P) ALARIA ESCULENTA (UNII: EJ9JK8J58D) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCO-GLYCERIDES (UNII: ISE9I7DNUG) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SORBITAN OLIVATE (UNII: MDL271E3GR) CETEARYL OLIVATE (UNII: 58B69Q84JO) METHYLCELLULOSE, UNSPECIFIED (UNII: Z944H5SN0H) COCONUT OIL (UNII: Q9L0O73W7L) STEARIC ACID (UNII: 4ELV7Z65AP) LUVISET 360 (UNII: 05BG6GY6YK) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) TOCOPHEROL (UNII: R0ZB2556P8) XANTHAN GUM (UNII: TTV12P4NEE) ALUMINUM OXIDE (UNII: LMI26O6933) BENZYL ALCOHOL (UNII: LKG8494WBH) SALICYLIC ACID (UNII: O414PZ4LPZ) SORBIC ACID (UNII: X045WJ989B) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69555-021-30 30 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 2 NDC:69555-021-60 60 mL in 1 TUBE; Type 0: Not a Combination Product 08/18/2021 3 NDC:69555-021-90 90 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 4 NDC:69555-021-32 946 mL in 1 TUBE; Type 0: Not a Combination Product 02/13/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/13/2021 Labeler - Stream2Sea, LLC (052920828) Establishment Name Address ID/FEI Business Operations Stream2Sea, LLC 052920828 manufacture(69555-020, 69555-021, 69555-030, 69555-031)