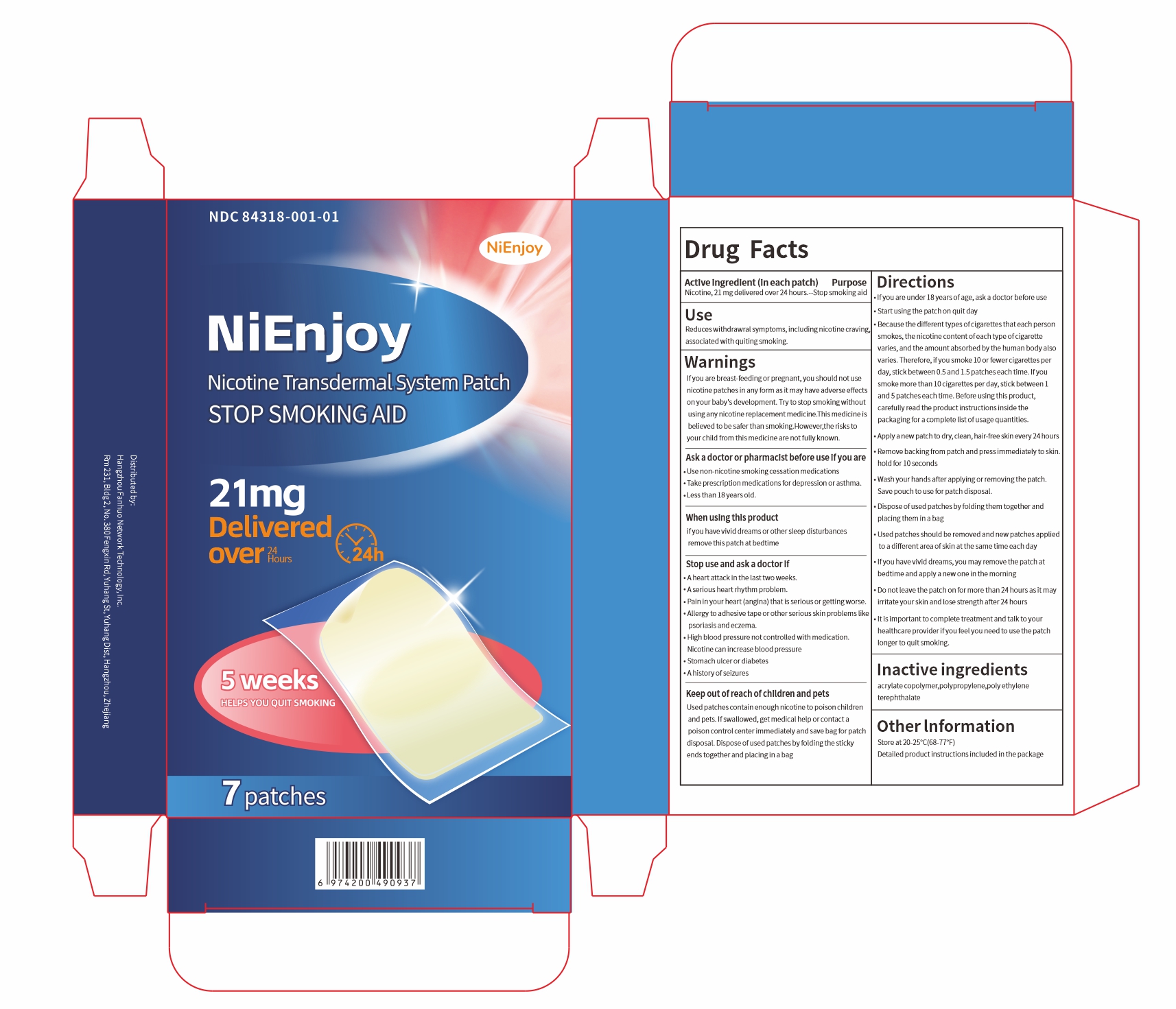
Label: NIENJOY NICOTINE TRANSDERMAL SYSTEM- nienjoy nicotine transdermal system patch patch
- NDC Code(s): 84318-001-01
- Packager: Hangzhou Fanhuo Network Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
If you are breast-feeding or pregnant,you should not use nicotine patches in any form asit may have adverse effects onyour baby's development. Try to stopsmoking without using any nicotine replacement medicine.This medicine is believed to be safer than smoking. However,the risks to your child from this medicine arenot fully known.
- DO NOT USE
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
Aheart attack in the last two weeks.
A serious heart rhythm problem.
Pain in your heart (angina) that is serious or getting worse.
Alergy to adhesive tape or other serious skin problemslike psoriasis and eczema.
High blood pressure not controlled with medication.Nicotine can increaseblood pressure
Stomach ulcer or diabetes
Ahistory of seizures
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
-If youare under 18 years of age,ask a doctor before use.
-Start using the patch on quit day.
-Because the different types of cigarettes that each person smokes, thenicotine content of each type of cigarette varies, and the amount absorbed by thehuman body also varies. Therefore, if you smoke 10 or fewer cigarettes per day,stick between 0.5and 1.5 patches each time. Ifyou smoke more than 10 cigarettes per day, stick between 1 and 5 patches each time. Before using this product, carefully read the product instructions inside the packaging for a complete list of usage quantities.
-Apply anewpatch to dry, dlean, hair-free skin every24 hours.
-Remove backing from patch and press immediately to skin.hold for 10 seconds.
-Wash your hands after applying or removing the patch.Save pouch to use for patch disposal.
- Dispose of used patches by folding them together and placing them in a bag.
-Used patches should be removed and new patches applied to a diflerent area of skin at the same time each day
- If you havevivid dreams, you may remove the patch at bedtime and apply a new one in themorning.
- Do not leave the patch on for more than 24 hours as it may irritate your skin and losestrength after 24 hours
-It is important to complete treatment and talk to your healthcare provider if you feel you need to use the patch longer to quit smoking.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIENJOY NICOTINE TRANSDERMAL SYSTEM
nienjoy nicotine transdermal system patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84318-001 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 21 mg in 24 h Inactive Ingredients Ingredient Name Strength POLYPROPYLENE (30000 MW) (UNII: T71QXI2O62) POLYETHYLENE TEREPHTHALATE (INTRINSIC VISCOSITY 0.40-0.70) (UNII: Y78AJD3DED) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84318-001-01 7 in 1 BOX 05/10/2024 1 24 h in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020076 05/10/2024 Labeler - Hangzhou Fanhuo Network Technology Co., Ltd. (713800244) Establishment Name Address ID/FEI Business Operations Hangzhou Fanhuo Network Technology Co., Ltd. 713800244 manufacture(84318-001)