Label: THERABIOGEN COLD AND FLU RELIEF NASAL- camelia sinensis, sambucus nigra, gelsemium sempervirens, bryonia alba, sulphur spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 51445-101-15 - Packager: THERABIOGEN
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 6, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
For best results, use at the first sign of cold or flu symptoms and continue to use for an additional 48 hours after symptoms subside
Adults and children 6 years of age and older (with adult supervision):
Remove cap and safety clip also see illustration on side carton and insert).
Hold with thumb at bottom of bottle and nozzle between your fingers
Before using the first tie, prime pump by depressing several times.
Place tip of nozzle just past nazal opening (approxiately 1/8").
While inside nasal opening, slightly angle nozzle outward.
Pump one to tw times into each nostril
After application, press lightly on outside of each nostril for about 5 seconds.
Wait at least 30 seconds before blowing nose
Use once every 4 hours
Children under 6 years of age: consult a doctor before use.
- INACTIVE INGREDIENT
-
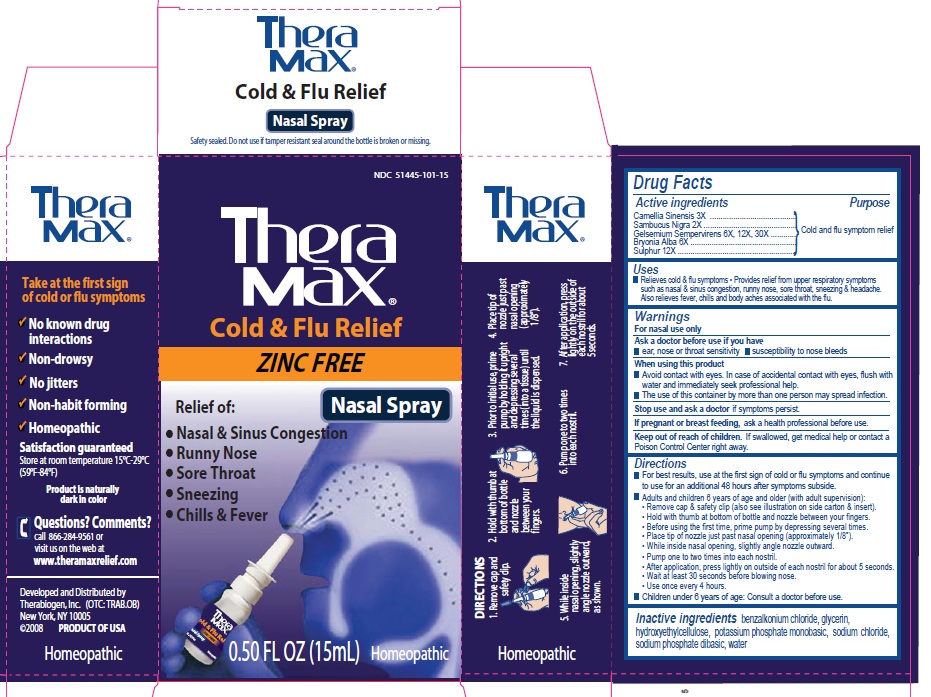
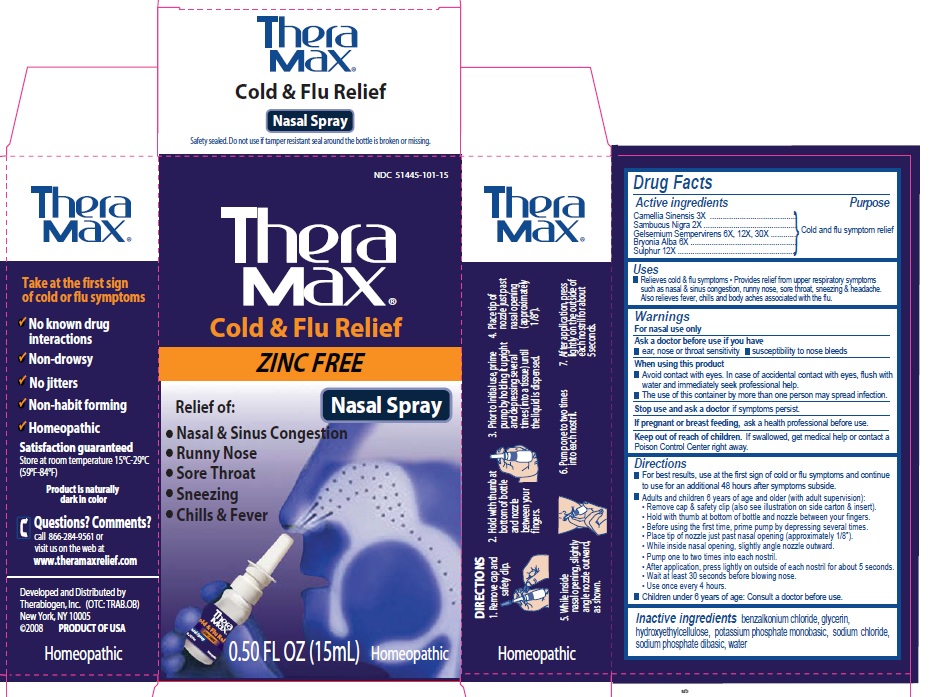
PRINCIPAL DISPLAY PANEL
Theramax
Take at the first sign of cold or flu symptoms
No known drug interactions
Non-drowsy
No jitters
Non-habit forming
Homeopathic
Satisfaction guaranteed
Store at room temperature 15C-29C (59F-84F)
Product is naturally dark in color
Questions? Comments?
call 866-284-9561 or visit us on the web at
www.theramaxrelief. com
Developed and distributed by
Therabiogen, Inc. (OTC: TRAB.OB)
New York, NY 10005
(c) 2008 PRODUCT OF USA
HOMEOPATHIC
Theramax
Cold and flu relief
ZINC FREE
Nasal Spray
Relief of:
Nasal and Sinus Congestion
Runny Nose
Sore Throat
Sneezing
Chills and Fever
0.50 fl oz (15 ml) Homeopathic
TheraMax
DIRECTIONS
1. Remove cap and safety clip
2. Hold with thumb at bottom and nozzle between your fingers
3. Prior to initial use, prime pump by holding it upright and depressing several ties (into a tissue) until the liquid is dispensed
4. Place tip of nozzle just past nasal opening (approximately 1/8")
5. While inside nasal opening, slightly angle nozzle outward, as shown
6. Pump one to two times into each nostril
7. After application, press lightly on the outside of each nostril for about 5 seconds.
-
PRINCIPAL DISPLAY PANEL
Active Ingredients:
Camellia simensis 3X,
Sambucus nigra 2X
Gelsemium sempervirens 6X, 12X, 30X
Bryonica Alba 6X
Sulphur 12X
Note: Prior to using TheraMax Cold and Flu Relief, read the entire package
(c) 2008 Developed and distributed by Therabiogen, Inc. (OTC: TRABE.OB)
New York, NY 10005
www.theramaxrelief.com
PRODUCT OF USA
TheraMax
Cold and Flu Relief
Zinc Free
Nasal Spary
0.50 fl oz (15 ml) Homeopathic
Do not use if tamper resistant seal is broken or missing.
Uses: Relieves cold and flu symptoms. Provides relief from upper respiratory symptoms such as nasal and sinus congestion, runny nose, sore throat, sneezing and headach. Also relieves fever, chills, ad body aches associated with the flu.
Questions or commeents:
Call 866-284-9561
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERABIOGEN COLD AND FLU RELIEF NASAL
camelia sinensis, sambucus nigra, gelsemium sempervirens, bryonia alba, sulphur sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51445-101 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) (GREEN TEA LEAF - UNII:W2ZU1RY8B0) GREEN TEA LEAF 3 [hp_X] in 15 mL SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 2 [hp_X] in 15 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 6 [hp_X] in 15 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 15 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 15 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51445-101-15 1 in 1 BOX 1 15 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/29/2010 Labeler - THERABIOGEN (831281733) Establishment Name Address ID/FEI Business Operations COSMEDX SCIENCE, INC 016437738 manufacture