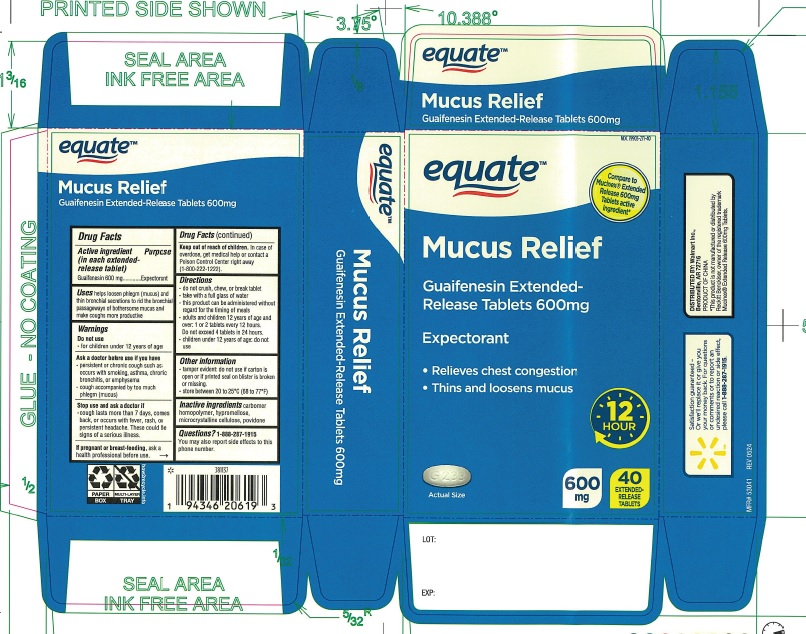
Label: GUAIFENESIN EXTENDED RELEASE 600 MG- guaifenesin tablet, extended release
- NDC Code(s): 79903-271-40
- Packager: Wal-Mart Stores, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(in each extended-release tablet)
- PURPOSE
- USE(S)
- WARNING
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- STOP USE AND ASK A DOCTOR IF
- IF PREGNANT OR BREAST-FEEDING,
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN EXTENDED RELEASE 600 MG
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-271 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 17mm Flavor Imprint Code G233 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-271-40 2 in 1 CARTON 10/22/2024 1 20 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209215 10/22/2024 Labeler - Wal-Mart Stores, Inc. (051957769) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(79903-271)