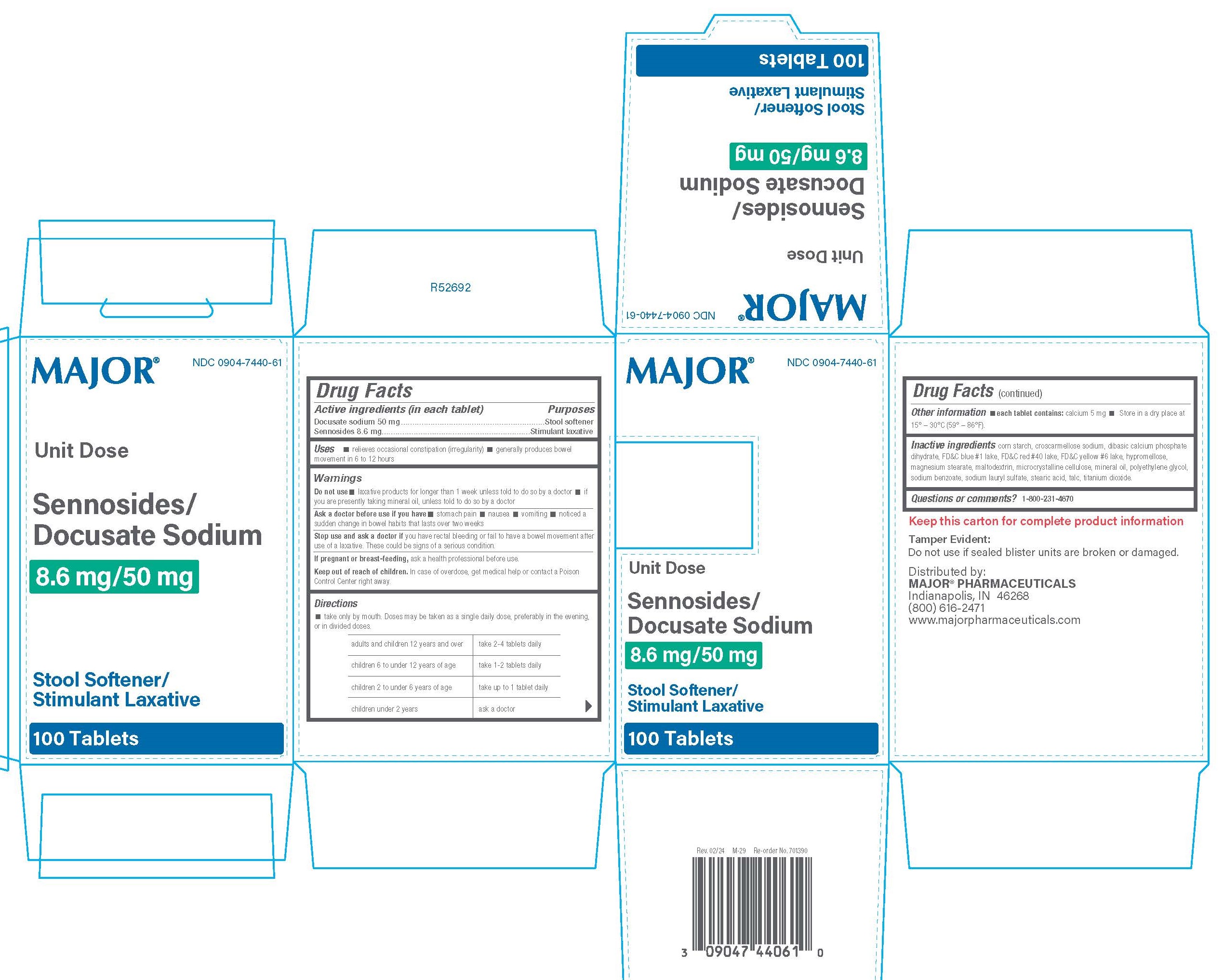
Label: SENNOSIDES, DOCUSATE SODIUM tablet, film coated
- NDC Code(s): 0904-7440-61
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
-
WARNINGS
- laxative products for longer than 1 week unless told to do so by a doctor
- if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over two weeks
-
Directions
- take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
adults and children 12 years and over take 2-4 tablets daily children 6 to under 12 years of age take 1-2 tablets daily children 2 to under 6 years of age take up to 1 tablet daily children under 2 years ask a doctor
- take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
corn starch, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue #1 lake, FD&C red #40 lake, FD&C yellow #6 lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil, polyethylene glycol, sodium benzoate, sodium lauryl sulfate, stearic acid, talc, titanium dioxide.
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENNOSIDES, DOCUSATE SODIUM
sennosides, docusate sodium tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STARCH, CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) STEARIC ACID (UNII: 4ELV7Z65AP) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C YELLOW NO. 6 ALUMINUM LAKE (UNII: GYP6Z2JR6Q) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) FD&C RED NO. 40 ALUMINUM LAKE (UNII: 6T47AS764T) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM BENZOATE (UNII: OJ245FE5EU) TALC (UNII: 7SEV7J4R1U) LIGHT MINERAL OIL (UNII: N6K5787QVP) Product Characteristics Color red Score no score Shape ROUND Size 10mm Flavor Imprint Code 49;0 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7440-61 10 in 1 BOX, UNIT-DOSE 05/01/2024 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 05/01/2024 Labeler - Major Pharmaceuticals (191427277)