Label: PHEXXI- lactic acid, l-, citric acid monohydrate, and potassium bitartrate gel
- NDC Code(s): 69751-100-01, 69751-100-03, 69751-100-12
- Packager: Evofem, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PHEXXI ® safely and effectively. See full prescribing information for PHEXXI.
PHEXXI (lactic acid, citric acid, and potassium bitartrate) vaginal gel
Initial U.S. Approval: 2020INDICATIONS AND USAGE
PHEXXI is a combination of lactic acid, citric acid, and potassium bitartrate indicated for the prevention of pregnancy in females of reproductive potential for use as an on-demand method of contraception. (1)
Limitations of Use: PHEXXI is not effective for the prevention of pregnancy when administered after intercourse.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Each pre-filled single-dose vaginal applicator delivers 5 grams of gel containing lactic acid (1.8%), citric acid (1%), and potassium bitartrate (0.4%). (3)
WARNINGS AND PRECAUTIONS
- Cystitis and Pyelonephritis: Avoid use in women with a history of recurrent UTI or urinary tract abnormalities (5.1)
ADVERSE REACTIONS
Most common adverse reactions (≥2%) were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, vulvovaginal discomfort, bacterial vaginosis, vaginal discharge, genital discomfort, dysuria, and vulvovaginal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Evofem at toll-free phone 1-833-EVFMBIO or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Timing of PHEXXI Use
2.3 Use of PHEXXI with Other Contraceptive Methods
2.4 Use of PHEXXI with Other Vaginal Products
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Cystitis and Pyelonephritis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
PHEXXI is indicated for the prevention of pregnancy in females of reproductive potential for use as an on-demand method of contraception.
Limitations of Use
PHEXXI is not effective for the prevention of pregnancy when administered after intercourse [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer one pre-filled applicator of PHEXXI (5 grams) vaginally immediately before or up to one hour before each act of vaginal intercourse. If more than one act of vaginal intercourse occurs within one hour, an additional dose must be applied. Five grams of PHEXXI contains 90 mg of lactic acid, 50 mg of citric acid, and 20 mg of potassium bitartrate.
2.2 Timing of PHEXXI Use
May use PHEXXI during any part of the menstrual cycle. May use PHEXXI as soon as it is safe to resume vaginal intercourse after childbirth, abortion, or miscarriage.
- 3 DOSAGE FORMS AND STRENGTHS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cystitis and Pyelonephritis
Among 2804 subjects who received PHEXXI in Studies 1 and 2, 0.36% (n=10) reported adverse reactions of cystitis, pyelonephritis, or other upper urinary tract infection (UTI). Of these, one case of pyelonephritis was considered serious and required hospitalization. Avoid use of PHEXXI in females of reproductive potential with a history of recurrent urinary tract infection or urinary tract abnormalities.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cystitis and Pyelonephritis [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of PHEXXI (pre-filled applicator with 5-gram dose) has been evaluated in two clinical trials (Study 1 and Study 2) in 2804 subjects (over 19,000 cycles of exposure). The racial/ethnic distribution was 66% White, 27% Black or African American, 2% Asian, 1% American Indian or Alaska Native, 0.3% Native Hawaiian or Pacific Islander, and 5% other; 32% of the study population was Hispanic. Study 1 included a one-year extension phase where 342 U.S. subjects were exposed to PHEXXI for 13 cycles.
Hypersensitivity Reaction
Of the 2804 PHEXXI-treated subjects in Studies 1 and 2, one subject reported a suspected drug hypersensitivity. Avoid PHEXXI use in females of reproductive potential with suspected hypersensitivity to the ingredients in PHEXXI.
The most common adverse reactions (≥10%) in the U.S. population in Studies 1 and 2 (n = 2480) were: vulvovaginal burning sensation (18.0%) and vulvovaginal pruritus (14.5%). The majority of these adverse reactions were mild and few led to discontinuation. Table 1 summarizes the most common adverse reactions (≥2%) reported by subjects using PHEXXI in the U.S.
Table 1. Adverse Reactions that Occurred in ≥ 2% of Subjects Who Used PHEXXI to Prevent Pregnancy (Studies 1 and 2 – U.S. population only) Adverse Reaction PHEXXI
(N=2480)
(%)- *
- Includes preferred terms (PT) vulvovaginal mycotic infection and vulvovaginal candidiasis.
- †
- Includes PTs urinary tract infection, streptococcal urinary tract infection, Escherichia urinary tract infection, and urinary tract infection bacterial.
- ‡
- Does not include PTs cystitis, kidney infection, and pyelonephritis [see Warnings and Precautions (5.1)].
Vulvovaginal Burning Sensation 18.0 Vulvovaginal Pruritus 14.5 Vulvovaginal Mycotic Infection* 9.1 Urinary Tract Infection† ‡ 9.0 Vulvovaginal Discomfort 9.0 Bacterial Vaginosis 8.4 Vaginal Discharge 5.5 Genital Discomfort 4.1 Dysuria 3.1 Vulvovaginal pain 2.1 Among subjects who used PHEXXI in Studies 1 and 2, 1.6% discontinued from the clinical trials due to an adverse reaction. The most common adverse reactions leading to study discontinuation were vulvovaginal burning sensation (0.7%); and vulvovaginal pruritus and vulvovaginal discomfort (0.1% each).
Adverse Reactions in Male Partners
Among male partners of subjects who used PHEXXI for contraception in Study 2, 9.8% (131 of 1330) reported symptoms of local discomfort (burning, itching, pain, and "other"). Of these local discomfort symptoms, 74.7% were mild, 21.4% were moderate, and 3.9% were severe. Two subjects discontinued participation in the study due to male partner symptoms.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There is no use for PHEXXI in pregnancy; therefore, discontinue PHEXXI during pregnancy. There are no data with the use of PHEXXI in pregnant women or animals. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
-
11 DESCRIPTION
PHEXXI (lactic acid, citric acid, and potassium bitartrate) is a vaginal gel.
PHEXXI is an off-white to tan in color gel of uniform consistency, containing three active ingredients: lactic acid, citric acid, and potassium bitartrate.
The structural formula for lactic acid is:
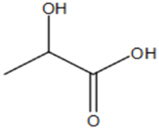
Lactic acid is designated chemically as 2-hydroxypropanoic acid with an empirical formula of C3H6O3 and a molecular weight of 90.08 g/mol.
The structural formula for citric acid is:
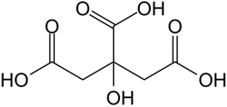
Citric acid is designated chemically as 2-hydroxypropane-1,2,3-tricarboxylic acid with an empirical formula of C6H8O7 and a molecular weight of 192.124 g/mol.
The structural formula for potassium bitartrate is:
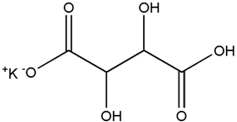
Potassium bitartrate is designated chemically as potassium; (2R, 3R)-2,3,4-trihydroxy-4-oxobutanoate with an empirical formula of KC4H5O6 and a molecular weight of 188.177 g/mol.
Each 5 gram dose is provided in a pre-filled single-dose applicator containing lactic acid USP (1.8% w/w), citric acid USP (1% w/w), and potassium bitartrate USP (0.4% w/w). Inactive ingredients present in the gel are: glycerin, alginic acid, xanthan gum, sodium hydroxide, benzoic acid, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In in vitro studies, Phexxi produced a normal vaginal pH range (pH 3.5 – 4.5) in the presence of semen. In clinical studies, post-coital testing demonstrated pH < 5 in the majority of subjects, and sperm motility reduction.
12.3 Pharmacokinetics
Pharmacokinetic studies in humans have not been performed. Systemic exposures of lactic acid, citric acid, and potassium bitartrate following vaginal administration of PHEXXI are not expected to lead to safety concerns.
In vitro studies with commonly used vaginal preparations (miconazole, metronidazole, tioconazole, and a product for maintaining normal vaginal pH) showed no significant effect on the pH or buffering capacity of PHEXXI.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of PHEXXI for the prevention of pregnancy was evaluated in a multi-center, open-label, single-arm clinical trial in the United States (AMP002; NCT03243305). The study enrolled females of reproductive potential 18 to 35 years of age with regular menstrual cycles (21 to 35 days). The median age was 27.8 years. The racial distribution was 70.6% White, 23.7% Black or African American, 2.5% Asian, 0.4% American Indian or Alaska Native, 0.2% Native Hawaiian or Pacific Islander, and 2.7% other. Subjects agreed to engage in at least 3 acts of heterosexual, vaginal intercourse per cycle. Subjects self-administered a 5 gram dose of PHEXXI intravaginally up to one hour before each episode of intercourse for up to 7 cycles.
The primary efficacy endpoint was the 7-cycle typical use cumulative pregnancy rate as derived by Kaplan-Meier life-table analysis. A total of 101 on-treatment pregnancies occurred in 1183 subjects contributing 4769 evaluable natural cycles. The 7-cycle cumulative pregnancy rate was 13.7% (95% CI: 10.0%, 17.5%), excluding cycles with back-up contraception, cycles <21 days or >35 days in length and cycles in which no intercourse was reported. The estimated Pearl Index, calculated based on data from the 7-cycle study, was 27.5 (95% CI: 22.4%, 33.5%).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PHEXXI (lactic acid, citric acid, and potassium bitartrate) vaginal gel is an off-white to tan color gel of uniform consistency containing lactic acid (1.8%), citric acid (1%), and potassium bitartrate (0.4%), supplied as individually wrapped 5 gram pre-filled single-dose vaginal applicators in sealed foil pouches along with a plunger, and are available as follows:
- NDC 69751-100-12
Box of 12 units - NDC 69751-100-03
Sample box of 3 units -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the Patient Information and FDA-approved patient labeling (Instructions for Use). Advise the patient:
- To intravaginally administer the contents of one pre-filled single-dose applicator of PHEXXI before each episode of vaginal intercourse and to administer an additional dose if intercourse does not occur within one hour of administration [see Dosage and Administration (2.1)].
- To consult their healthcare provider for severe or prolonged genital irritation [see Adverse Reactions (6.1)].
- To discontinue PHEXXI if they develop a local hypersensitivity reaction [see Adverse Reactions (6.1)].
- To contact their healthcare provider if experiencing urinary tract symptoms [see Warnings and Precautions (5.1)].
- That PHEXXI does not protect against HIV infection and other sexually transmitted infections.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION PHEXXI® (FEX ee)
(lactic acid, citric acid, and potassium bitartrate) vaginal gel
For Vaginal Use OnlyThis Patient Information has been approved by the U.S. Food and Drug Administration Issued: June 2023 What is PHEXXI? - PHEXXI is a prescription medicine used to prevent pregnancy in females who can become pregnant and choose to use an on-demand method of birth control.
- PHEXXI is not effective at preventing pregnancy when used after vaginal sex.
How well does PHEXXI work? Your chance of getting pregnant depends on how well you follow the directions for using PHEXXI. The better you follow the directions, the less chance you have of getting pregnant. It is very important that you follow the directions carefully each time you have vaginal sex. PHEXXI does not protect against HIV infection or other sexually transmitted infections (STIs). Before using PHEXXI, tell your healthcare provider about all of your medical conditions, including if you: - are pregnant or think you are pregnant. PHEXXI is not for use in pregnant women.
- are breastfeeding or plan to breastfeed. It is not known if PHEXXI passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. How should I use PHEXXI? - See the Instructions for Use that come with PHEXXI for detailed instructions on the right way to use PHEXXI.
- Use PHEXXI exactly as your healthcare provider tells you to use it.
- PHEXXI must be used before vaginal sex.
- PHEXXI comes as a pre-filled single-dose vaginal applicator.
- Insert 1 PHEXXI pre-filled applicator into your vagina and use PHEXXI within 1 hour before each time you have vaginal sex. If you do not have vaginal sex within 1 hour of using PHEXXI, you must insert a new PHEXXI pre-filled applicator.
- If you have vaginal sex more than 1 time within 1 hour, you must use a new PHEXXI pre-filled applicator.
- PHEXXI may be used at any time during the menstrual cycle.
- PHEXXI may be used as soon as your healthcare provider tells you it is safe for you to have vaginal sex after childbirth, abortion, or miscarriage.
- PHEXXI may be used with hormonal contraceptives; and latex, polyurethane and polyisoprene condoms. PHEXXI may be used with a vaginal diaphragm. Avoid using PHEXXI with contraceptive vaginal rings.
- PHEXXI may be used with other medicines used in the vagina to treat infections including miconazole, metronidazole and tioconazole.
What are the possible side effects of PHEXXI? PHEXXI may cause serious side effects, including: - Bladder infection (cystitis) and acute kidney infection (pyelonephritis). Urinary tract infections are common but can also be serious. You should not use PHEXXI if you have a history of urinary tract infections that keep coming back or other problems with your urinary tract. Call your healthcare provider if you have burning with urination or other signs and symptoms of a urinary tract infection such as: burning feeling when passing urine, urine that looks cloudy, pain in the pelvis, or back pain.
- Allergic reactions. Avoid using PHEXXI if you are a female who can become pregnant and are allergic to lactic acid, citric acid, potassium bitartrate or any of the ingredients in PHEXXI; or your sexual partners are allergic to any of the ingredients in PHEXXI". Stop using PHEXXI if you have a local vulvovaginal reaction.
The most common side effects of PHEXXI include: - vaginal burning
- vaginal itching
- vaginal yeast infection
- discomfort around the vaginal area
- bacterial vaginosis
- vaginal discharge
- discomfort in the genital area
- pain while passing urine
These are not all the possible side effects of PHEXXI. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store PHEXXI? - Store PHEXXI at room temperature between 68°F to 77°F (20°C to 25°C).
- Store PHEXXI in the original foil pouch.
Keep PHEXXI and all medicines out of the reach of children. General information about the safe and effective use of PHEXXI. Medicines are sometimes used for purposes other than those listed in a Patient Information leaflet. Do not use PHEXXI for a condition for which it was not prescribed. Do not give PHEXXI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about PHEXXI that is written for health professionals. What are the ingredients in PHEXXI? Active ingredients: lactic acid, citric acid, and potassium bitartrate Inactive ingredients: glycerin, alginic acid, xanthan gum, sodium hydroxide, benzoic acid, and purified water For more information, go to www.phexxi.com or call 1-833-EVFMBIO. Manufactured for Evofem, Inc., a wholly owned subsidiary of Evofem Biosciences, Inc. ©2023 Evofem, Inc. All rights reserved. Unless otherwise indicated, all trademarks used herein are the property of Evofem Biosciences, Inc. -
INSTRUCTIONS FOR USE
PHEXXI® (FEX ee)
(lactic acid, citric acid, and potassium bitartrate) vaginal gelFor Vaginal Use Only
These Instructions for Use contain information on how to use PHEXXI vaginal gel. Make sure that you read, understand, and follow the Instructions for Use before using PHEXXI and each time you get a refill. There may be new information.
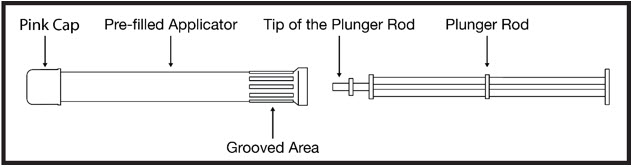
Contents:
- Each box contains either 3 or 12 foil pouches.
- Each foil pouch contains a pre-filled applicator and plunger rod (see Figure A).
- Each pre-filled applicator contains 1 dose of PHEXXI for 1-time use (single use).
Important Information You Need to Know Before Using PHEXXI
- Use 1 dose of PHEXXI within 1 hour before you have vaginal sex.
- A new PHEXXI pre-filled applicator must be used each time you have vaginal sex. If you have vaginal sex more than 1 time within 1 hour, a new PHEXXI pre-filled applicator must be used.
Prepare to Use PHEXXI
Keep the pre-filled applicator and plunger rod in the foil pouch until you are ready to use PHEXXI.
Step 1: Wash Your Hands
- Wash your hands with soap and water before opening the foil pouch.
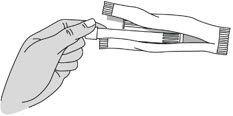
Step 2: Remove the Pre-filled Applicator and Plunger Rod from the Foil Pouch
- Remove the pre-filled applicator and plunger rod from the foil pouch (see Figure B).
Figure B Important: Do not remove the pink cap until instructed in Step 4.
Insert PHEXXI Gel
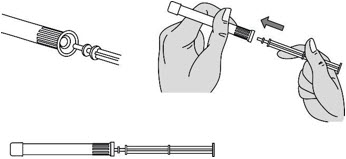
Step 3: Insert the Plunger Rod
- Gently and slowly insert the plunger rod into the pre-filled applicator. Push until you feel the tip of the plunger rod connect to the inside of the pre-filled applicator (see Figure C).
- Do not push hard or continue to push after the tip of the plunger rod connects to the inside of the pre-filled applicator. This could cause the gel to go into the pink cap.
- Use a new pre-filled applicator if the gel goes into the pink cap.
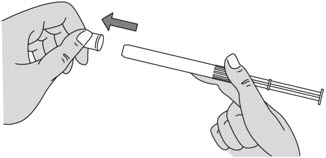
Figure C Step 4: Remove the Pink Cap
- After the plunger rod is connected to the pre-filled applicator, remove the pink cap from the pre-filled applicator (see Figure D).
- The extra space between the gel and the end of the pre-filled applicator is normal.
- The pre-filled applicator is now ready for use.
Figure D Step 5: Insert the PHEXXI Pre-filled Applicator into the Vagina
- Hold the pre-filled applicator at the grooved area closest to the plunger rod (see Figure E).
- Gently insert the pre-filled applicator into the vagina as far as it will comfortably go while you continue to hold it by the grooved area firmly. This can be done sitting with your knees apart, lying on your back with your knees bent (see Figure F), or while standing with your feet apart or knees bent.
Figure E Figure F Step 6: Insert PHEXXI Gel
- While the pre-filled applicator is inserted in your vagina, use your index finger to push the plunger rod down until it stops. This is to make sure you receive the entire dose of PHEXXI (see Figure G).
- It is normal for a small amount of gel to be left in the applicator. You will still get the right dose.
Figure G Step 7: Remove the Used PHEXXI Pre-filled Applicator
- Gently remove the plunger rod and pre-filled applicator from the vagina (see Figure H) and throw away (dispose of) the used pre-filled applicator.
- PHEXXI should be used within 1 hour before each time you have vaginal sex. Use a new pre-filled applicator if you do not have vaginal sex within 1 hour of inserting PHEXXI gel and you still plan to have vaginal sex.
Figure H Disposing of PHEXXI
Step 8: Throw Away (Dispose of) the Used PHEXXI Pre-filled Applicator
- Used PHEXXI pre-filled applicators and caps should be disposed of in the trash. The cap may be a potential choking hazard.
Storing PHEXXI
- Store PHEXXI at room temperature between 68°F to 77°F (20°C to 25°C).
- Store PHEXXI in the original foil pouch.
Keep PHEXXI and all medicines out of the reach of children.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
For more information, including full prescribing information and information on patient safety, go to www.phexxi.com or call 1-833-EVFMBIO.
Manufactured for Evofem, Inc., a wholly owned subsidiary of Evofem Biosciences, Inc.
©2023 Evofem, Inc. All rights reserved.Issued: June 2023
- PRINCIPAL DISPLAY PANEL - 12 Applicator Box
-
INGREDIENTS AND APPEARANCE
PHEXXI
lactic acid, l-, citric acid monohydrate, and potassium bitartrate gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69751-100 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 90 mg in 5 g CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 50 mg in 5 g POTASSIUM BITARTRATE (UNII: NPT6P8P3UU) (TARTARIC ACID - UNII:W4888I119H) POTASSIUM BITARTRATE 20 mg in 5 g Product Characteristics Color white (off-white to tan) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69751-100-12 12 in 1 BOX 09/01/2020 1 NDC:69751-100-01 5 g in 1 APPLICATOR; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:69751-100-03 3 in 1 BOX 05/24/2021 2 NDC:69751-100-01 5 g in 1 APPLICATOR; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208352 08/01/2020 Labeler - Evofem, Inc. (832466119)