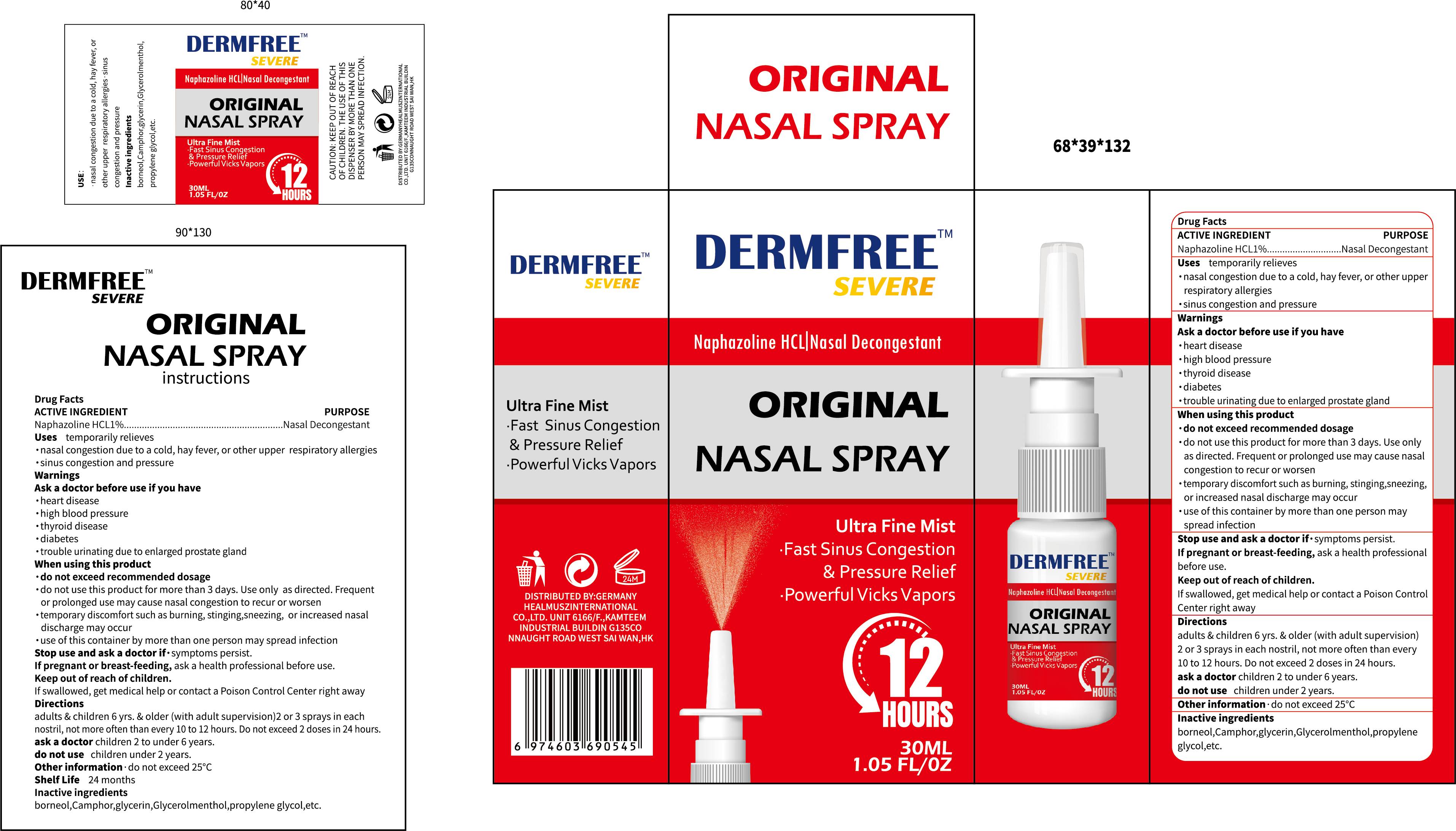
Label: DERMFREE ORIGINAL NASAL- naphazoline hcl 1%original nasal spray
- NDC Code(s): 84010-011-01
- Packager: Jiangxi Hemei Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMFREE ORIGINAL NASAL
naphazoline hcl 1%original nasal sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84010-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHAZOLINE HYDROCHLORIDE (UNII: MZ1131787D) (NAPHAZOLINE - UNII:H231GF11BV) NAPHAZOLINE HYDROCHLORIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIGLYCERIN (UNII: 3YC120743U) MENTHOL (UNII: L7T10EIP3A) BORNEOL (UNII: M89NIB437X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84010-011-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/29/2024 Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) Establishment Name Address ID/FEI Business Operations Jiangxi Hemei Pharmaceutical Co., Ltd 724892056 manufacture(84010-011)