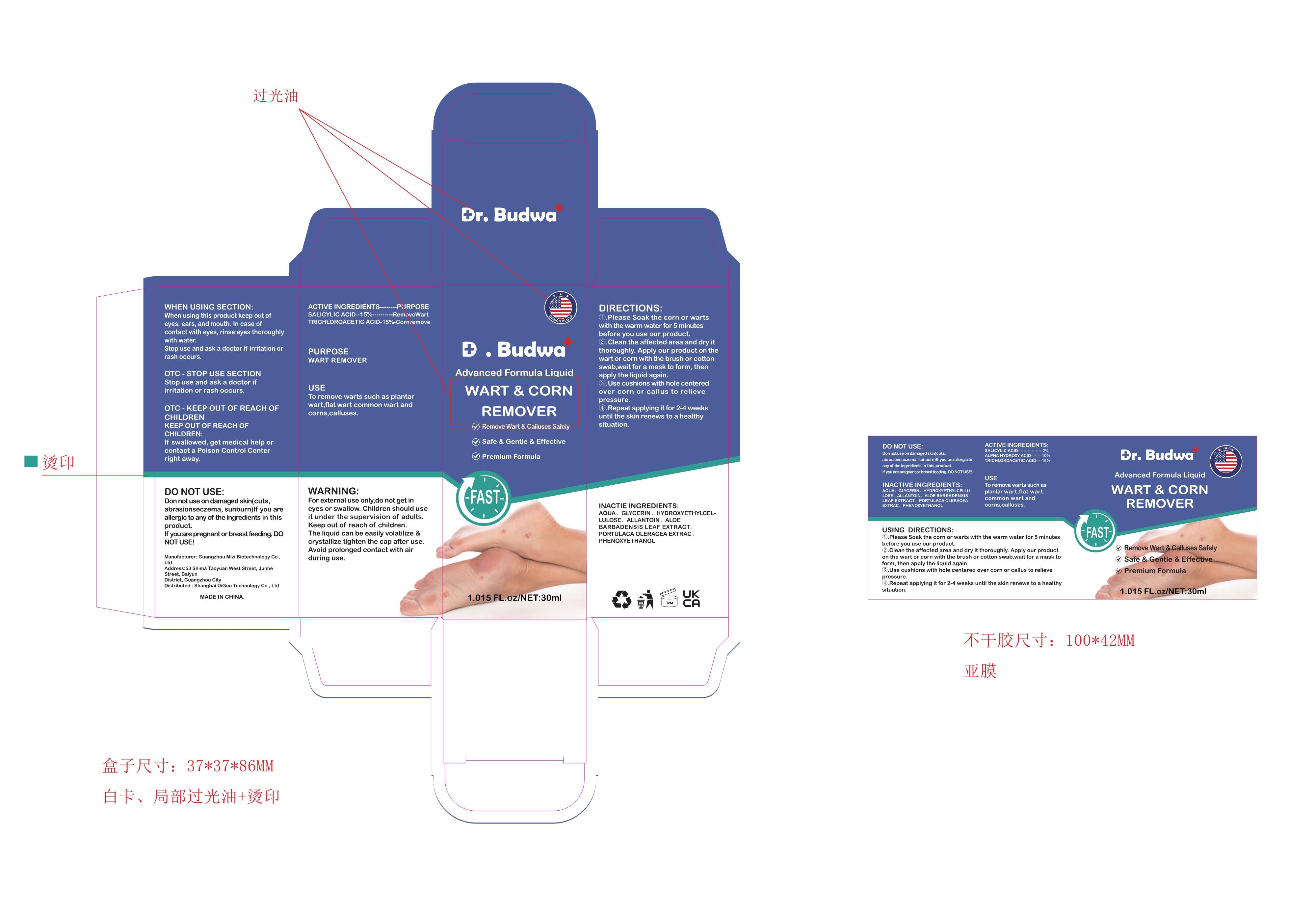
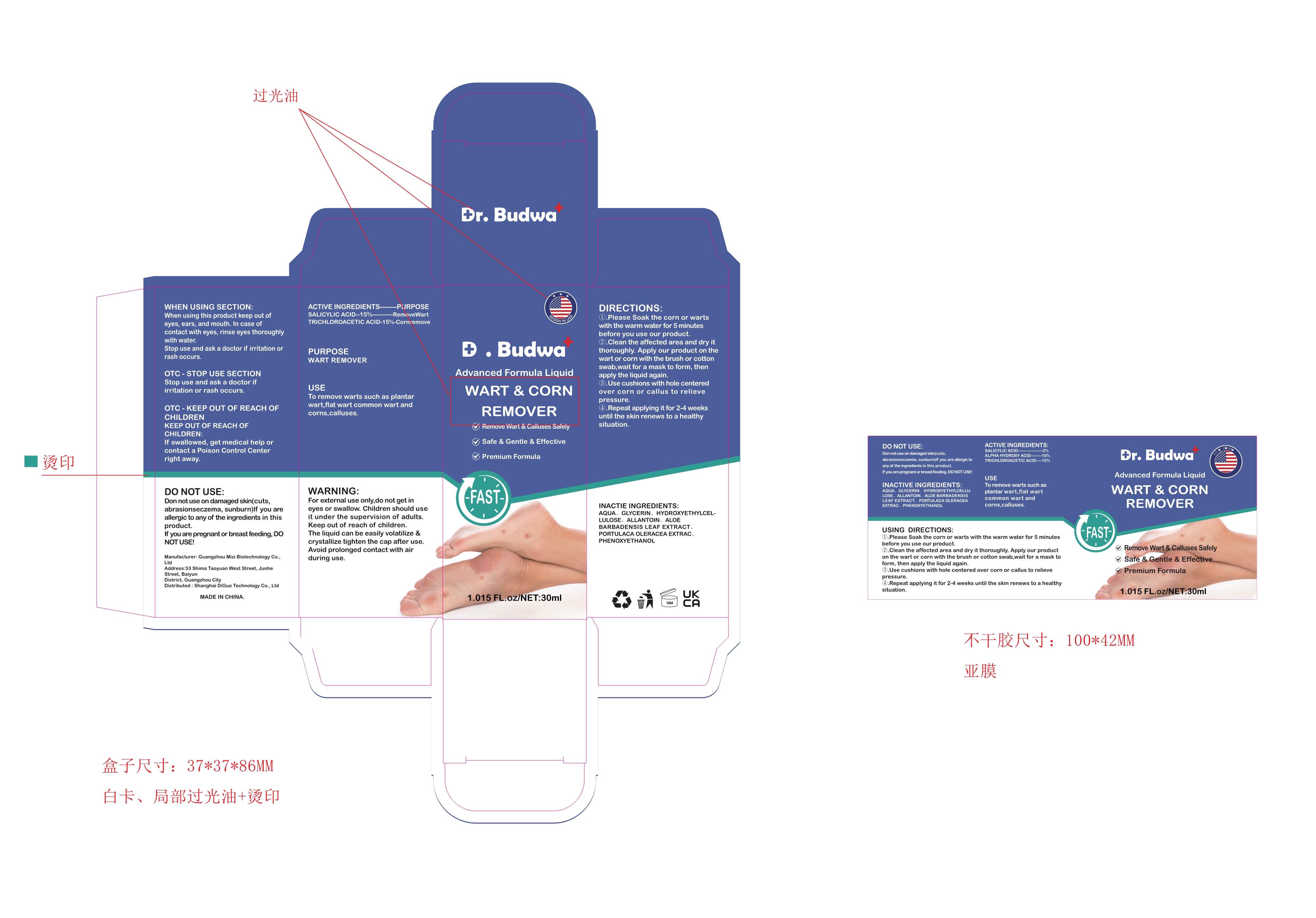
Label: WART REMOVER LIQUID liquid
- NDC Code(s): 84251-002-01
- Packager: Shanghai DiGuo Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
①.Please Soak the corn or warts with the warm water for 5 minutes before you use our product.
②.Clean the affected area and dry it thoroughly. Apply our product on the wart or corn with the brush or cotton
swab,wait for a mask to form, then apply the liquid again.
③.Use cushions with hole centered over corn or callus to relieve pressure.
④.Repeat applying it for 2-4 weeks until the skin renews to a healthy situation. - Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WART REMOVER LIQUID
wart remover liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84251-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRICHLOROACETIC ACID (UNII: 5V2JDO056X) (TRICHLOROACETIC ACID - UNII:5V2JDO056X) TRICHLOROACETIC ACID 15 g in 100 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 15 g in 100 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) PURSLANE (UNII: M6S840WXG5) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84251-002-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 04/29/2024 Labeler - Shanghai DiGuo Technology Co., Ltd (975242493) Establishment Name Address ID/FEI Business Operations 975242493 975242493 manufacture(84251-002)