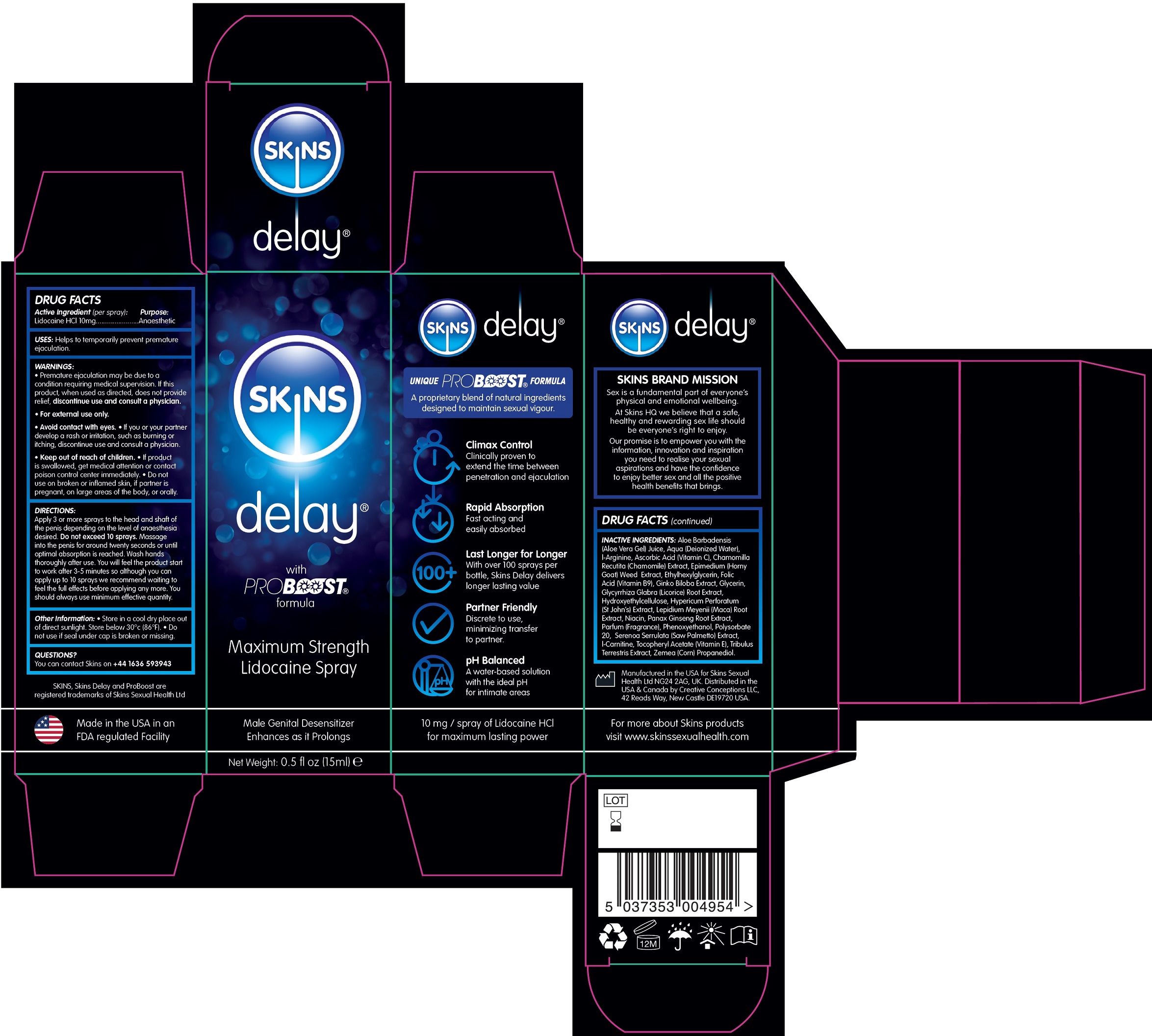
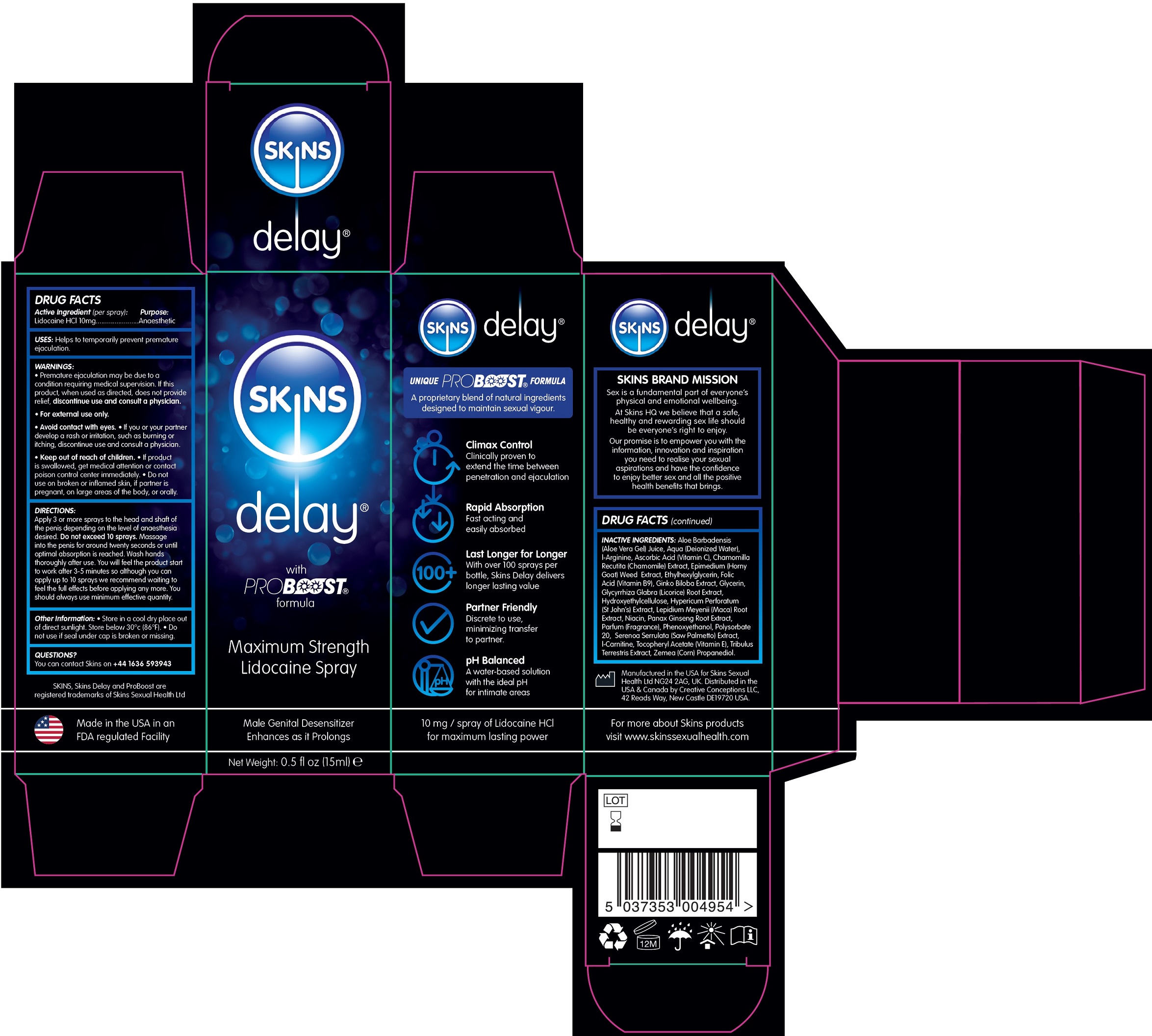
Label: SKINS DELAY MAXIMUM STRENGTH LIDOCAINE- lidocaine hydrochloride solution
- NDC Code(s): 81923-199-00
- Packager: SKINS SEXUAL HEALTH LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredient (per spray):
- USES:
-
WARNINGS:
- Premature ejaculation may be due to a condition requiring medical supervision. If this product, when used as directed, does not provide relief, discontinue use and consult a physician.
- For external use only.
- Avoid contact with eyes.
- If you or your partner develop a rash or irritation, such as burning or itching, discontinue use and consult a physician.
-
DIRECTIONS:
Apply 3 or more sprays to the head and shaft of the penis depending on the level of anaesthesia desired. . Massage into the penis for around twenty seconds or until optimal absorption is reached. Wash hands thoroughly after use. You will feel the product start to work after 3-5 minutes so although you can apply up to 10 sprays we recommend waiting to feel the full effects before applying any more. You should always use minimum effective quantity. Do not exceed 10 sprays
- Other Information:
- QUESTIONS?
-
INACTIVE INGREDIENTS:
Aloe Barbadensis (Aloe Vera Gel) Juice, Aqua (Deionized Water), l-Arginine, Ascorbic Acid (Vitamin C), Chamomilla Recutita (Chamomile) Extract, Epimedium (Horny Goat) Weed Extract, Ethylhexylglycerin, Folic Acid (Vitamin B9), Ginko Biloba Extract, Glycerin, Glycyrrhiza Glabra (Licorice) Root Extract, Hydroxyethylcellulose, Hypericum Perforatum (St John’s) Extract, Lepidium Meyenii (Maca) Root Extract, Niacin, Panax Ginseng Root Extract, Parfum (Fragrance), Phenoxyethanol, Polysorbate 20, Serenoa Serrulata (Saw Palmetto) Extract, l-Carnitine, Tocopheryl Acetate (Vitamin E), Tribulus Terrestris Extract, Zemea (Corn) Propanediol.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SKINS DELAY MAXIMUM STRENGTH LIDOCAINE
lidocaine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81923-199 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARGININE (UNII: 94ZLA3W45F) ASCORBIC ACID (UNII: PQ6CK8PD0R) CHAMOMILE (UNII: FGL3685T2X) EPIMEDIUM GRANDIFLORUM TOP (UNII: 137PC46F89) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FOLIC ACID (UNII: 935E97BOY8) GINKGO (UNII: 19FUJ2C58T) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) HYPERICUM PERFORATUM WHOLE (UNII: XK4IUX8MNB) LEPIDIUM MEYENII ROOT (UNII: HP7119212T) NIACIN (UNII: 2679MF687A) ASIAN GINSENG (UNII: CUQ3A77YXI) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) SAW PALMETTO (UNII: J7WWH9M8QS) LEVOCARNITINE (UNII: 0G389FZZ9M) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIBULUS TERRESTRIS WHOLE (UNII: 4X4HLN92OT) CORN (UNII: 0N8672707O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81923-199-00 1 in 1 BOX 05/25/2021 1 15 mL in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/25/2021 Labeler - SKINS SEXUAL HEALTH LIMITED (221947744)