Label: MUCORSAN- mucor racemosus capsule
- NDC Code(s): 73479-021-02
- Packager: sanPharmacy Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Indications
- Dosage
- ACTIVE INGREDIENT
-
Warnings
If pregnant or breast-feeding, ask a health professional before use.
______________________________
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
______________________________
When using this product
▪ do not use more than directed
______________________________
If symptoms persist more than 5 days, contact a licensed practitioner.
______________________________
Tamper Evident: Do not use product if tamper evident strip is broken. To report adverse events, contact MEDsan Inc. at 1-855-462-6334. - Tamper Evident
- SPL UNCLASSIFIED SECTION
- STORAGE AND HANDLING
- Liability statement
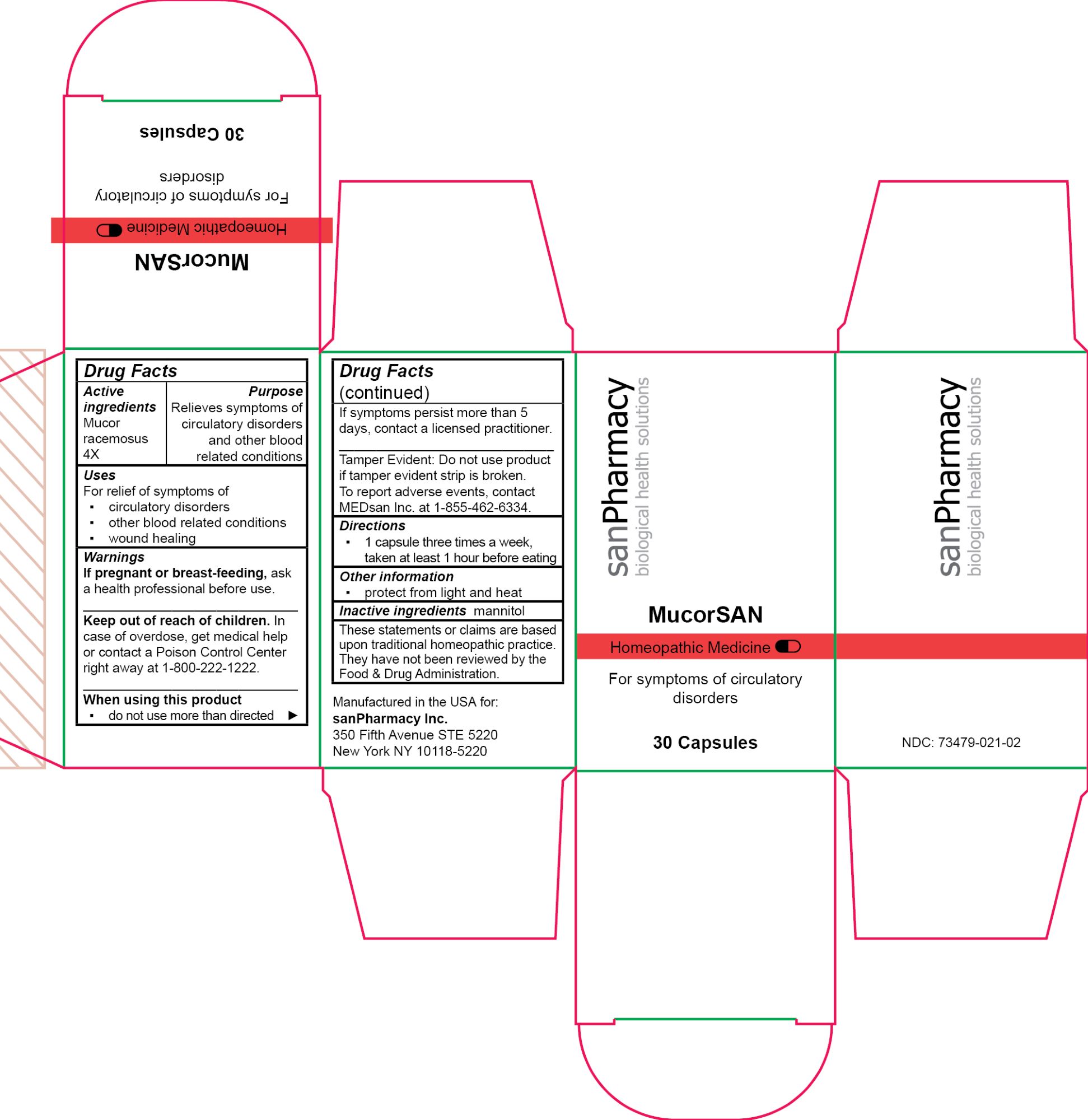
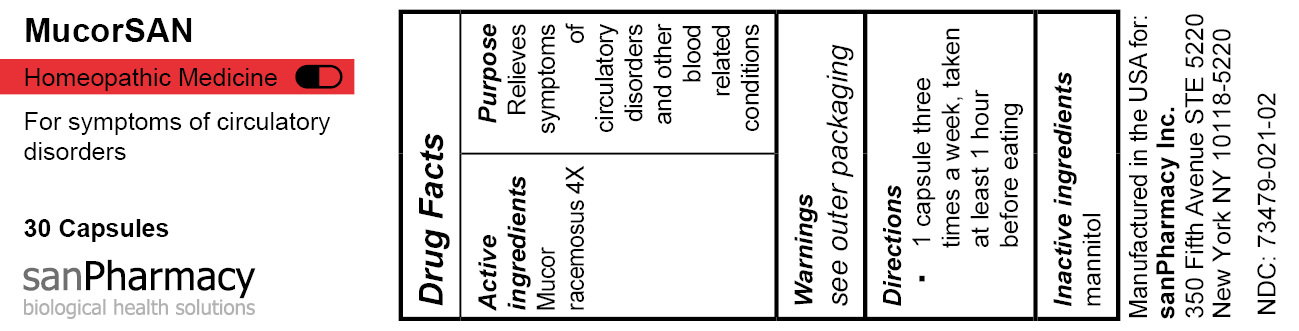
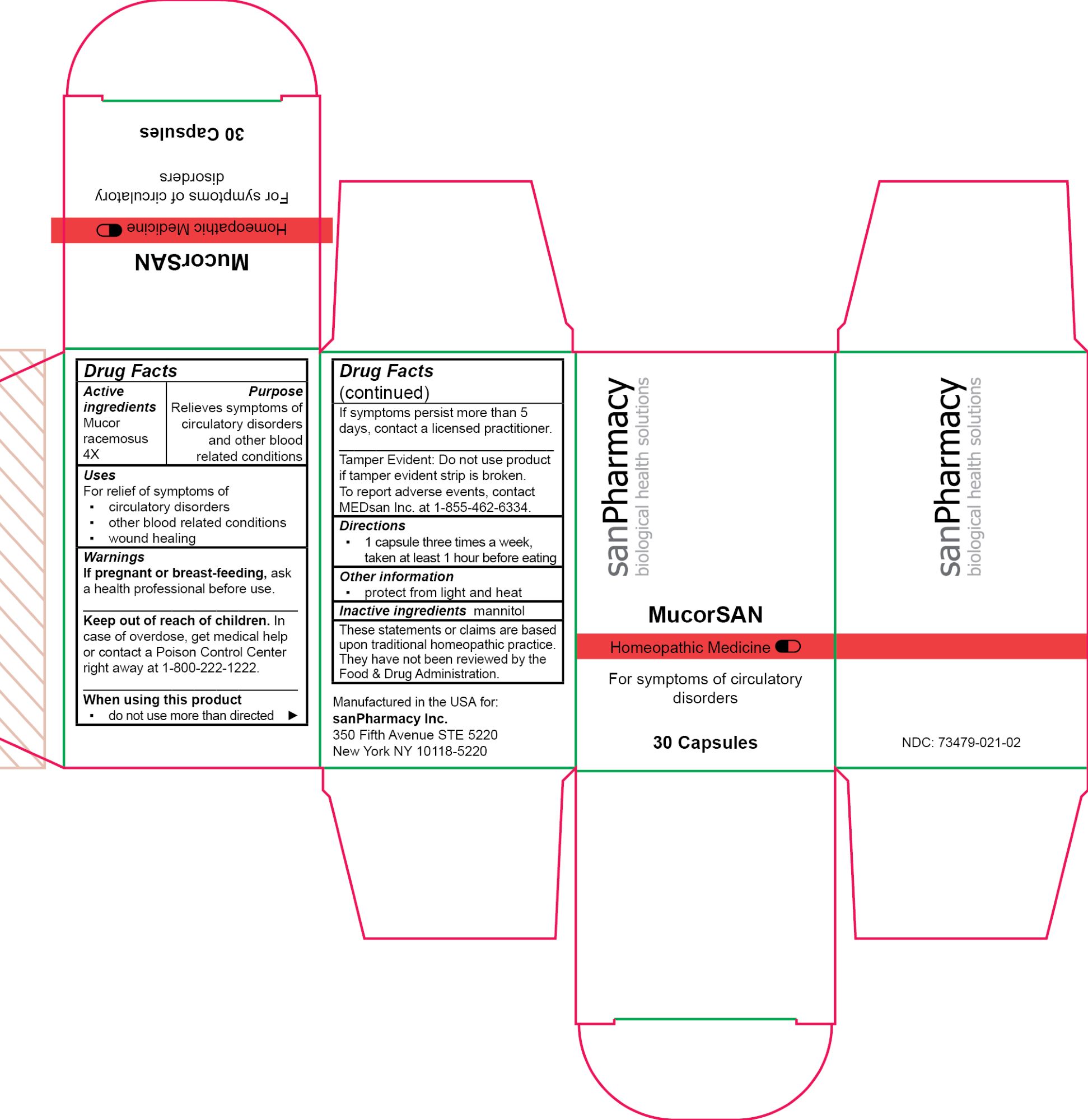
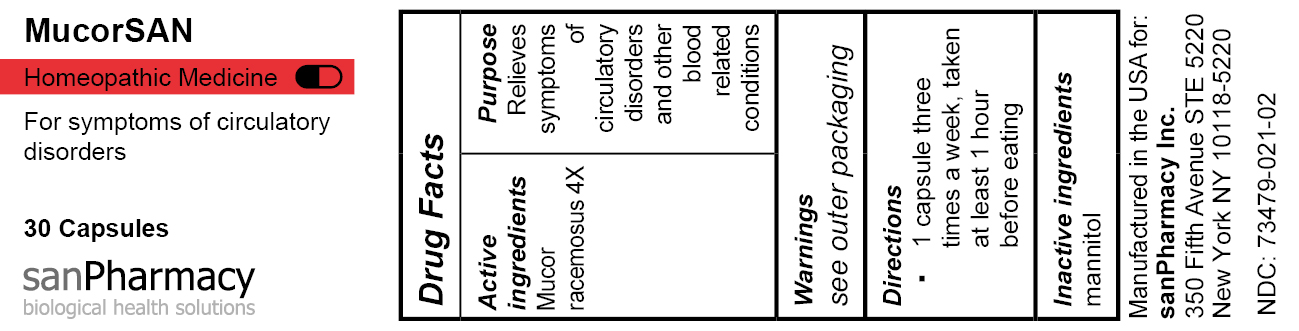
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MUCORSAN
mucor racemosus capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73479-021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MUCOR RACEMOSUS (UNII: 17RH99LQ7G) (MUCOR RACEMOSUS - UNII:17RH99LQ7G) MUCOR RACEMOSUS 4 [hp_X] Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73479-021-02 1 in 1 CARTON 04/25/2024 1 30 in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/25/2024 Labeler - sanPharmacy Inc. (117235152) Establishment Name Address ID/FEI Business Operations MEDsan Inc. 004326784 manufacture(73479-021)

 Capsules
Capsules