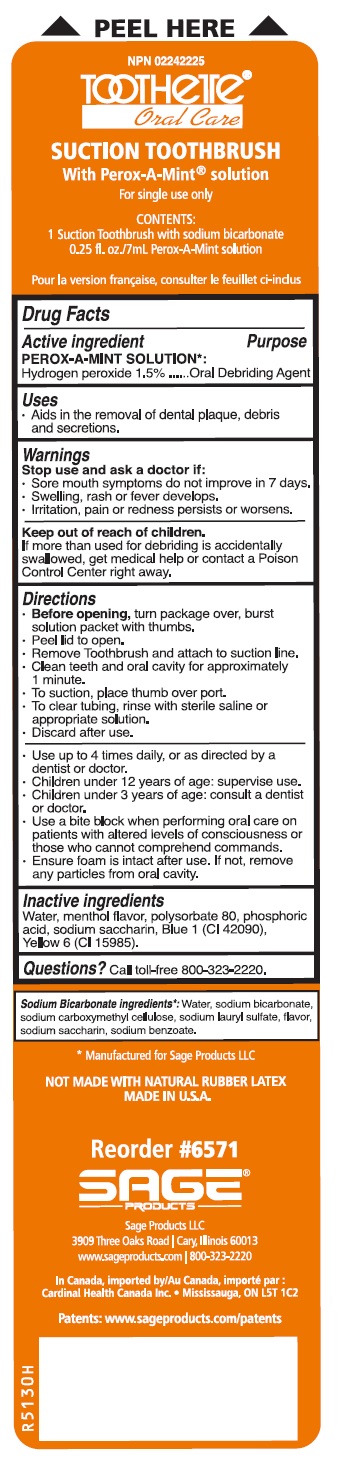
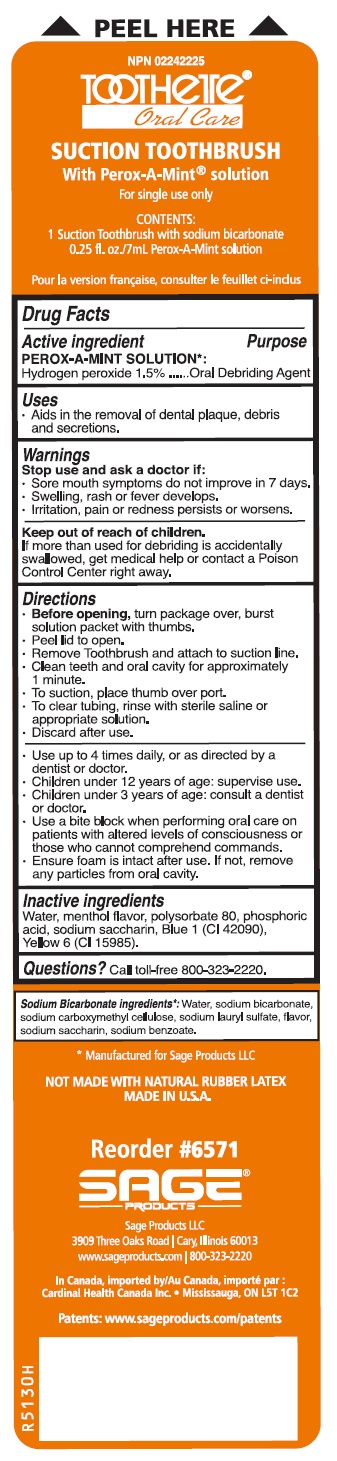
Label: SUCTION SWAB SYSTEM WITH PEROX-A-MINT SOLUTION - SUCTION SWAB AND SUCTION TOOTHBRUSH- hydrogen peroxide kit
SUCTION SWAB SYSTEM WITH PEROX-A-MINT SOLUTION - SUCTION SWAB- hydrogen peroxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 53462-553-60, 53462-650-60 - Packager: Sage Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Uses
- INDICATIONS & USAGE
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
Directions
Suction Swab
- Use as directed on individual packages
- Apply Mouth Moisturizer as needed.
- Discard after use.
- Suction Swabs and Applicator Swabs are intended for single oral use only.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Suction Swab and Suction Toothbrush
- Use as directed on individual packages
- Apply Mouth Moisturizer as needed.
- Discard after use.
- Suction Toothbrushes, Suction Swabs and Applicator Swabs are intended for single oral use only.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
- Inactive Ingredients
- QUESTIONS
- Suction Swab System with Perox-A-Mint Solution - Suction Swab
- Suction Swab System with Perox-A-Mint Solution - Suction Swab and Suction Toothbrush
-
INGREDIENTS AND APPEARANCE
SUCTION SWAB SYSTEM WITH PEROX-A-MINT SOLUTION - SUCTION SWAB AND SUCTION TOOTHBRUSH
hydrogen peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-553 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-553-60 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 06/30/2007 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 14 g Part 2 1 Part 3 8 PACKET 56 mL Part 1 of 3 MOUTH MOISTURIZER
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR XYLITOL (UNII: VCQ006KQ1E) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR WATER (UNII: 059QF0KO0R) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) INGR SPEARMINT OIL (UNII: C3M81465G5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 2 of 3 SODIUM BICARBONATE
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR SODIUM LAURYL SULFATE (UNII: 368GB5141J) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR WATER (UNII: 059QF0KO0R) INGR SACCHARIN SODIUM (UNII: SB8ZUX40TY) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 3 of 3 PEROX-A-MINT SOLUTION
hydrogen peroxide mouthwashProduct Information Item Code (Source) NDC:53462-075 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 in 1 KIT 1 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/30/2007 SUCTION SWAB SYSTEM WITH PEROX-A-MINT SOLUTION - SUCTION SWAB
hydrogen peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-650 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-650-60 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 06/28/2002 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 14 g Part 2 1 Part 3 6 PACKET 42 mL Part 1 of 3 MOUTH MOISTURIZER
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR XYLITOL (UNII: VCQ006KQ1E) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR WATER (UNII: 059QF0KO0R) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) INGR SPEARMINT OIL (UNII: C3M81465G5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 2 of 3 SODIUM BICARBONATE
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR SODIUM LAURYL SULFATE (UNII: 368GB5141J) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR WATER (UNII: 059QF0KO0R) INGR SACCHARIN SODIUM (UNII: SB8ZUX40TY) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 3 of 3 PEROX-A-MINT SOLUTION
hydrogen peroxide mouthwashProduct Information Item Code (Source) NDC:53462-075 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 in 1 KIT 1 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/28/2002 Labeler - Sage Products LLC (054326178) Registrant - Sage Products LLC (054326178)