Label: MINERAL LP FOUNDATION COTTON- mineral lp foundation cream
- NDC Code(s): 84179-205-01
- Packager: JENTRY KELLEY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Actives
- Purpose
- Use
- Warnings
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Apply liberally 15 minutes before sun exposure. Use a water-resistant sunscreen if swimming or sweating. Reapply at least every 2 hours, Children under 6 months: ask a doctor.
Sun Protection Measures: Spending time in the sun increases risk of skin cancer and early aging. To decrease
this risk, regularly use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun especially from 10:00 a.m. - 2:00 p.m. Wear long sleeved shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Cyclopentasiloxane, Aqua/Water/Eau, Dimethicone Crosspolymer, Octyldodecyl Neopentanoate, Butylene Glycol, Cetyl PEG/PPG-10/1 Dimethicone, Polyglyceryl-4 Isostearate, Aluminum Hydroxide, Stearic Acid, Dimethicone, Hexyl Laurate, Silica, Tocopherol, Tetrahexyldecyl Ascorbate, Sodium Hyaluronate, PEG/PPG-18/18 Dimethicone, Phytantriol, Nylon-12, Sodium Chloride, Nylon-12 Fluorescent Brightener 230 Salt, Polyvinylalcohol Crosspolymer, Octyldodecanol, Magnesium Chloride, Potassium Chloride, Zinc Chloride , Lysine, Methicone, Triethoxycaprylylsilane, Disodium EDTA, Hexylene Glycol, Caprylyl Glycol, Phenoxyethanol, Potassium Sorbate,Mica ,Titanium Dioxide (CI 77891), Iron Oxides (CI 77492), Iron Oxides (CI 77491), Iron Oxides (CI 77499)
- Other Information
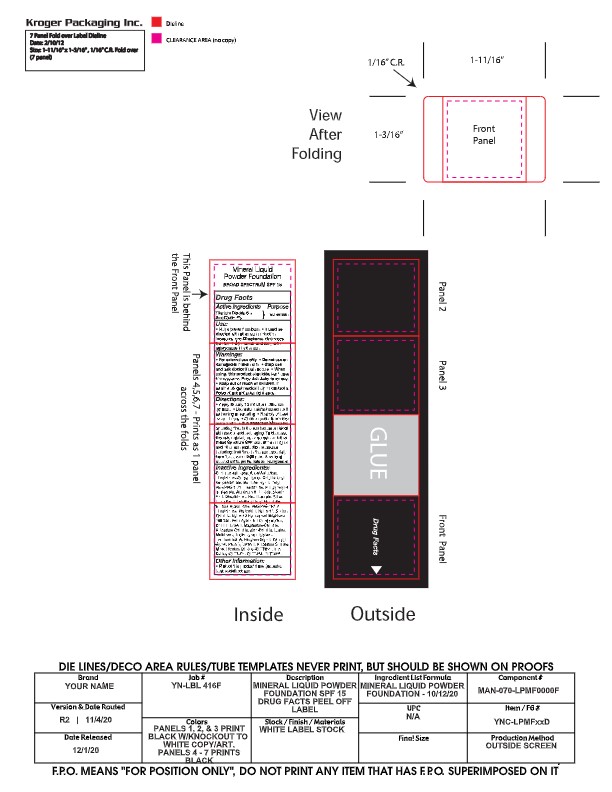
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL LP FOUNDATION COTTON
mineral lp foundation creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84179-205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 3 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 6 g in 30 mL Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POTASSIUM CHLORIDE (UNII: 660YQ98I10) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) DIMETHICONE (UNII: 92RU3N3Y1O) DISODIUM 4,4'-BIS((4-ANILINO-6-((2-CARBAMOYLETHYL)(2-HYDROXYETHYL)AMINO)-S-TRIAZIN-2-YL)AMINO)-2,2'-STILBENEDISULFONATE (UNII: 30I2S866LK) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ZINC CHLORIDE (UNII: 86Q357L16B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) LYSINE (UNII: K3Z4F929H6) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) WATER (UNII: 059QF0KO0R) HEXYL LAURATE (UNII: 4CG9F9W01Q) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) METHICONE (20 CST) (UNII: 6777U11MKT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) NYLON-12 (UNII: 446U8J075B) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) TOCOPHEROL (UNII: R0ZB2556P8) PHYTANTRIOL (UNII: 8LVI07A72W) OCTYLDODECANOL (UNII: 461N1O614Y) EDETATE DISODIUM (UNII: 7FLD91C86K) HEXYLENE GLYCOL (UNII: KEH0A3F75J) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84179-205-01 1 in 1 CARTON 08/01/2011 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2011 Labeler - JENTRY KELLEY (040036679) Registrant - MANA Products, Inc. (078870292) Establishment Name Address ID/FEI Business Operations MANA Products, Inc 078870292 manufacture(84179-205) Establishment Name Address ID/FEI Business Operations MANA Products,Inc 032870270 manufacture(84179-205)