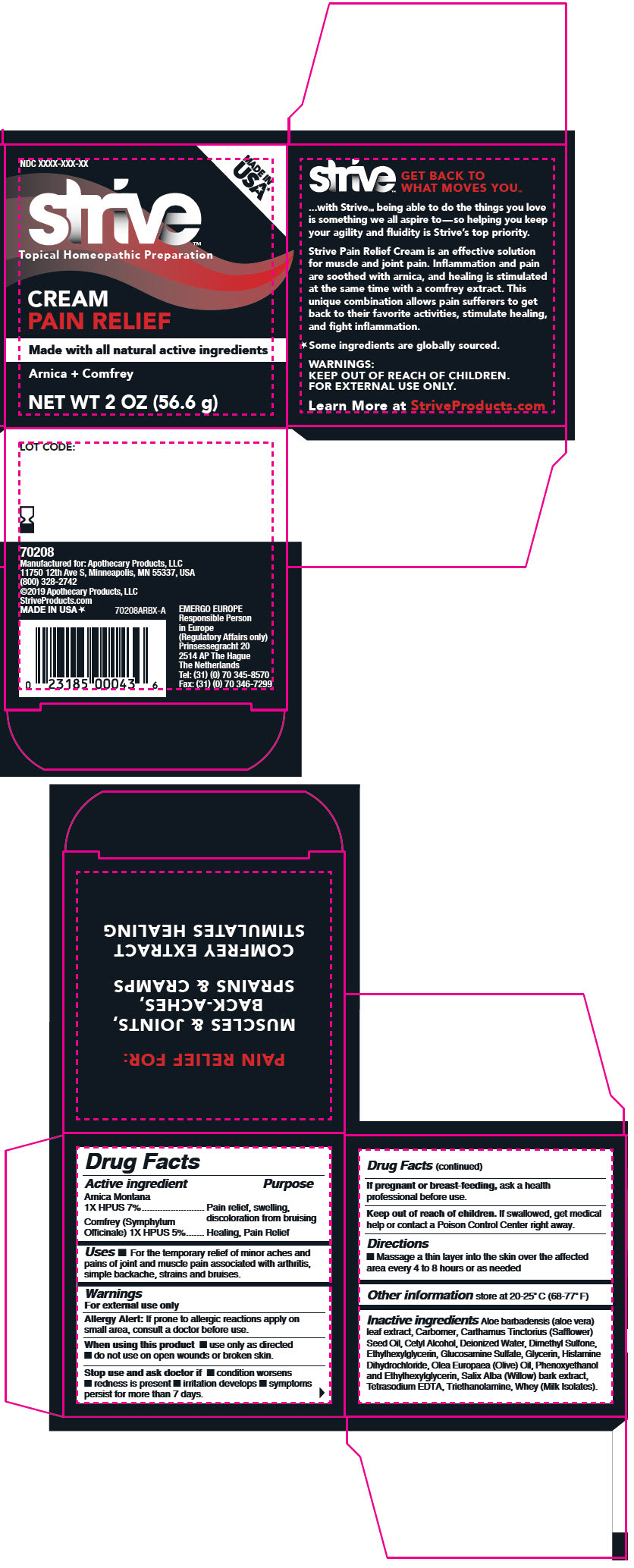
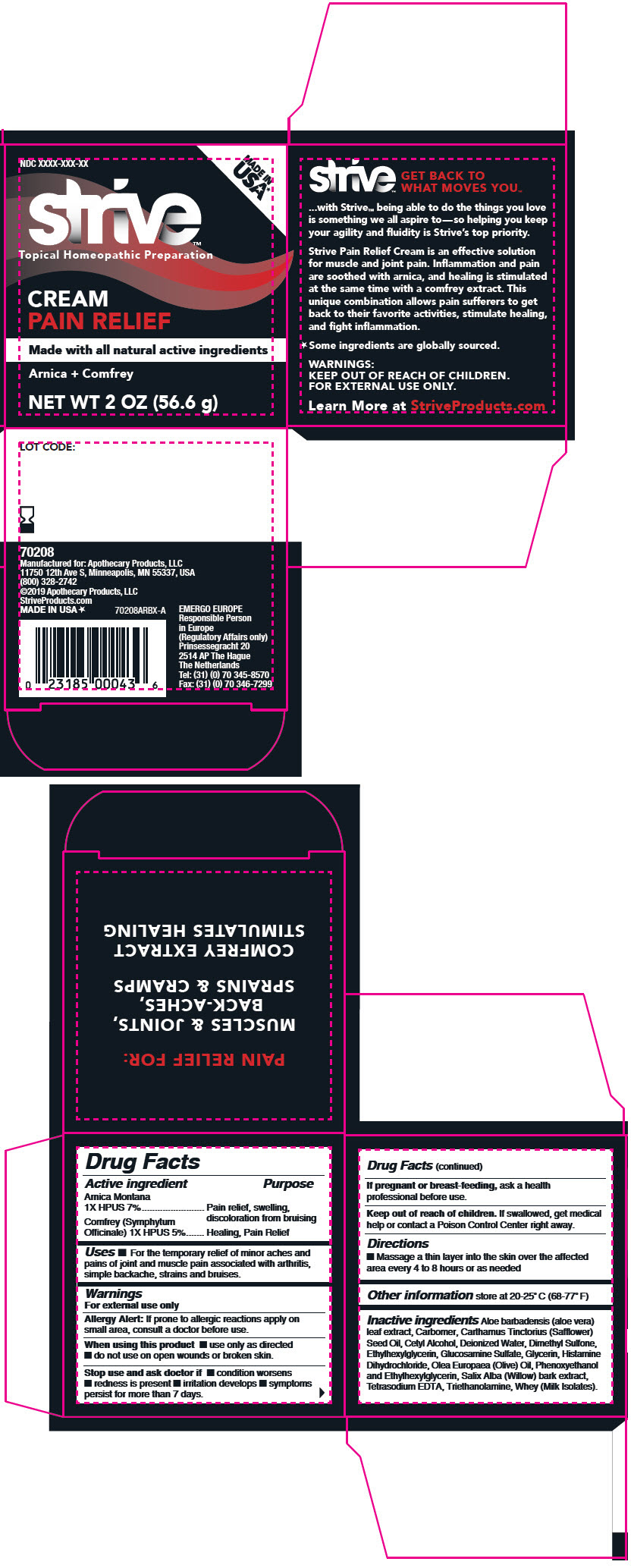
Label: STRIVE PAIN RELIEF- arnica montana flower and comfrey leaf cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 63374-002-01 - Packager: Steuart Custom Manufacturing
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 3, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aloe barbadensis (aloe vera) leaf extract, Carbomer, Carthamus Tinctorius (Safflower) Seed Oil, Cetyl Alcohol, Deionized Water, Dimethyl Sulfone, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Histamine Dihydrochloride, Olea Europaea (Olive) Oil, Phenoxyethanol and Ethylhexylglycerin, Salix Alba (Willow) bark extract, Tetrasodium EDTA, Triethanolamine, Whey (Milk Isolates).
- PRINCIPAL DISPLAY PANEL - 56.6 g Jar Carton
-
INGREDIENTS AND APPEARANCE
STRIVE PAIN RELIEF
arnica montana flower and comfrey leaf creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63374-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Arnica Montana Flower (UNII: OZ0E5Y15PZ) (Arnica Montana Flower - UNII:OZ0E5Y15PZ) Arnica Montana Flower 1 [hp_X] in 1 g Comfrey Leaf (UNII: DG4F8T839X) (Comfrey Leaf - UNII:DG4F8T839X) Comfrey Leaf 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Cetyl Alcohol (UNII: 936JST6JCN) Glycerin (UNII: PDC6A3C0OX) Safflower oil (UNII: 65UEH262IS) Ethylhexylglycerin (UNII: 147D247K3P) Phenoxyethanol (UNII: HIE492ZZ3T) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Salix Alba Bark (UNII: 205MXS71H7) Dimethyl Sulfone (UNII: 9H4PO4Z4FT) GLUCOSAMINE SULFATE POTASSIUM CHLORIDE (UNII: 15VQ11I66N) Water (UNII: 059QF0KO0R) Histamine Dihydrochloride (UNII: 3POA0Q644U) Carbomer Copolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: 809Y72KV36) Trolamine (UNII: 9O3K93S3TK) Whey (UNII: 8617Z5FMF6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63374-002-01 1 in 1 CARTON 04/15/2019 1 56.6 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 04/15/2019 Labeler - Steuart Custom Manufacturing (116952121) Establishment Name Address ID/FEI Business Operations Steuart Custom Manufacturing 116952121 MANUFACTURE(63374-002)