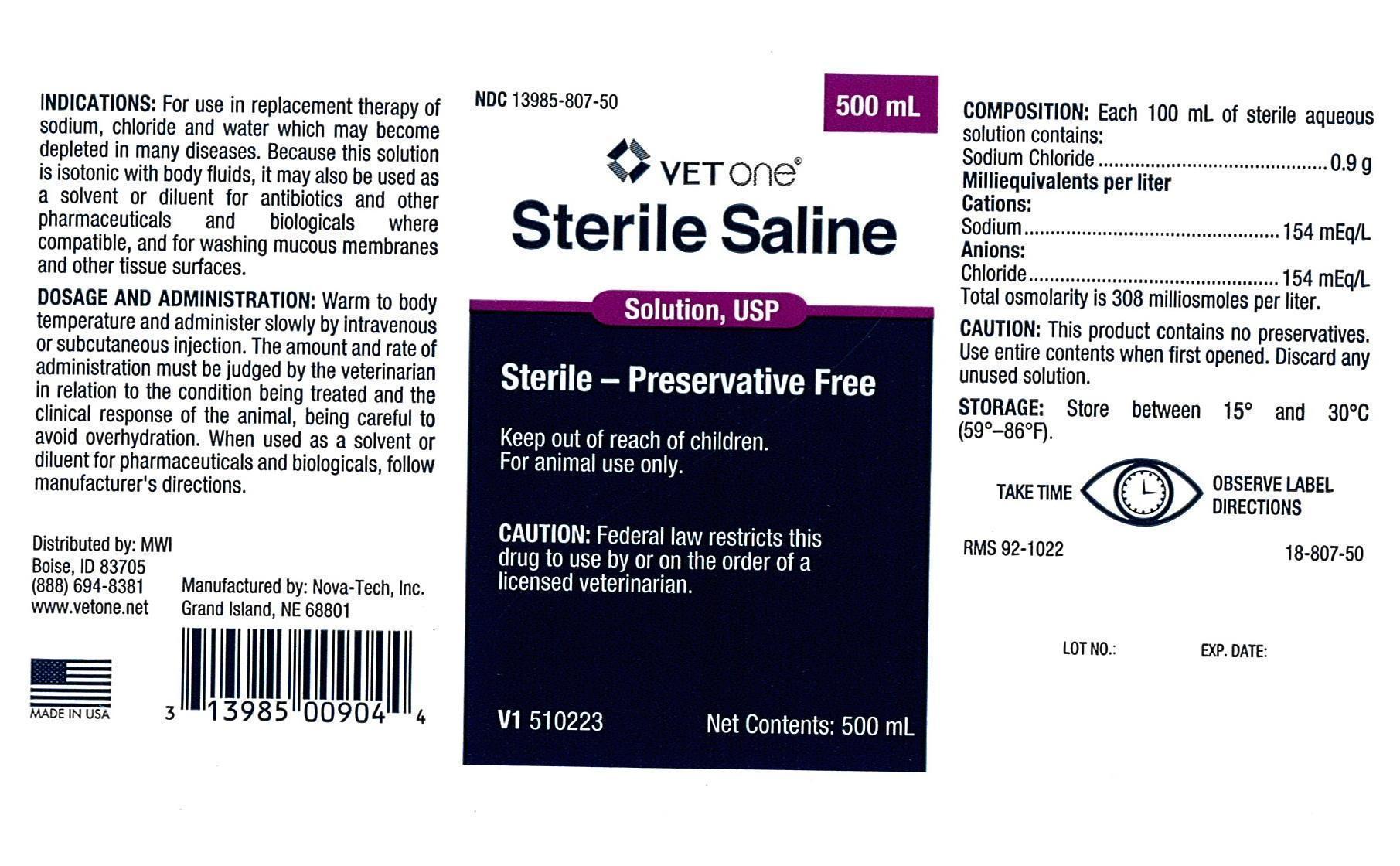
Label: STERILE SALINE- sodium chloride injection, solution
- NDC Code(s): 13985-807-25, 13985-807-50, 13985-807-60
- Packager: MWI (VetOne)
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 19, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS:
For use in replacement therapy of sodium, chloride and water which may become depleted in many diseases. Because this solution is isotonic with body fluids, it may also be used as a solvent or diluent for antibiotics and other pharmaceuticals and biologicals where compatible, and for washing mucous membranes and other tissue surfaces.
-
DOSAGE AND ADMINISTRATION:
Warm to body temperature and administer slowly by intravenous or subcutaneous injection. The amount and rate of administration must be judged by the veterinarian in relation to the condition being treated and the clinical response of the animal, being careful to avoid overhydration. When used as a solvent or diluent for pharmaceuticals and biologicals, follow manufacturer's directions.
- VETERINARY INDICATIONS
- CAUTION:
-
COMPOSITION:
Each 100 mL of sterile aqueous solution contains:
Sodium Chloride ..................................0.9 g
Milliequivalents per liter
Cations:
Sodium .............................................154 mEq/L
Anions:
Chloride.............................................154 mEq/L
Total osmolarity is 308 milliosmoles per liter.
- CAUTION:
- STORAGE:
- PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STERILE SALINE
sodium chloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-807 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37) Sodium Chloride 0.9 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-807-25 250 mL in 1 BOTTLE, PLASTIC 2 NDC:13985-807-50 500 mL in 1 BOTTLE, PLASTIC 3 NDC:13985-807-60 1000 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/17/2011 Labeler - MWI (VetOne) (019926120) Registrant - MWI (VetOne) (019926120) Establishment Name Address ID/FEI Business Operations Nova-Tech, Inc. 196078976 manufacture Establishment Name Address ID/FEI Business Operations Morton Salt 137658043 api manufacture