Label: TUSSIN MULTI SYMPTOM COLD CF ADULT- dextromethorphan hbr, guaifenesin, phenylephrine liquid
- NDC Code(s): 68788-8147-0
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 0904-6537
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 10 mL)
- Purposes
- Uses
-
Warnings
Do not use
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- diabetes
- •
- thyroid
- •
- trouble urinating due to an enlarged prostate gland
- •
- cough that occurs with too much phlegm ( mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are
taking any other oral nasal decongestant or stimulant.
-
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided. Do not use any other dosing device.
- •
- keep dosing cup with product
- •
- mL = milliliter
- •
- this adult product is not intended for use in children under 12 years of age
- •
- adult and children 12 years and over: 10 mL every 4 hours
- •
- children under 12 years: do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
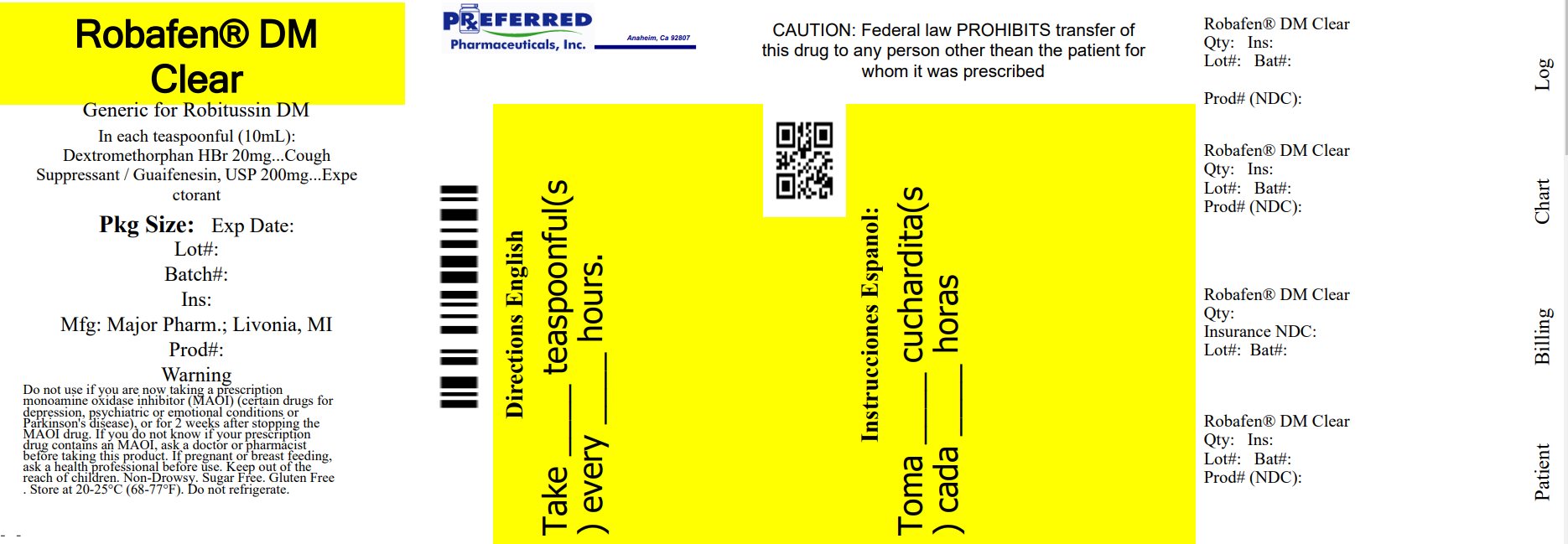
Principal Display Panel
ROBAFEN
CF MULTI-SYMPTOM COLD
Dextromethorphan HBr, 20 mg / COUGH SUPPRESSANT
Guaifenesin, 200 mg / EXPECTORANT
Phenylephrine HCl, 10 mg / NASAL DECONGESTANT
PEAK COLD
Relieves:
- •
- Cough
- •
- Mucus
- •
- Nasal Congestion
Non-Drowsy
COMPARE TO the active ingredients in ROBITUSSIN® PEAK COLD MULTI-SYMPTOM COLD CF*
FOR ADULTS
For Ages 12 Years and Over
Alcohol-Free
FL OZ (mL)
Dosing Cup Included
*This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of Robitussin® Peak Cold Multi-Symptom Cold CF.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by:
MAJOR PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152
- Package Label
-
INGREDIENTS AND APPEARANCE
TUSSIN MULTI SYMPTOM COLD CF ADULT
dextromethorphan hbr, guaifenesin, phenylephrine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-8147(NDC:0904-6537) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 10 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) FD&C RED NO. 40 (UNII: WZB9127XOA) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8147-0 1 in 1 BOX 09/01/2022 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/01/2022 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-8147)