Label: IZERVAY- avacincaptad pegol injection
- NDC Code(s): 82829-002-01, 82829-002-99
- Packager: Astellas Pharma US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated February 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IZERVAY safely and effectively. See full prescribing information for IZERVAY.
IZERVAY™ (avacincaptad pegol intravitreal solution)
Initial U.S. Approval: 2023INDICATIONS AND USAGE
IZERVAY is a complement inhibitor indicated for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD) (1).
DOSAGE AND ADMINISTRATION
The recommended dose for IZERVAY is 2 mg (0.1 mL of 20 mg/mL solution) administered by intravitreal injection to each affected eye once monthly (approximately 28 ± 7 days) for up to 12 months (2.2).
DOSAGE FORMS AND STRENGTHS
Intravitreal solution: 20 mg/mL in a single-dose vial (3).
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions were conjunctival hemorrhage (13%), increased IOP (9%), blurred vision (8%) and neovascular age-related macular degeneration (7%) (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Astellas Pharma US, Inc. at 1-800-707-4479 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Recommended Dosage
2.3 Preparation for Administration
2.4 Injection Procedure
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Ocular or Periocular Infections
4.2 Active Intraocular Inflammation
5 WARNINGS AND PRECAUTIONS
5.1 Endophthalmitis and Retinal Detachments
5.2 Neovascular AMD
5.3 Increase in Intraocular Pressure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.2 Recommended Dosage
The recommended dose for IZERVAY is 2 mg (0.1 mL of 20 mg/mL solution) administered by intravitreal injection to each affected eye once monthly (approximately every 28 ± 7 days) for up to 12 months.
2.3 Preparation for Administration
Important information you should know before you begin:
- •
- Read all the instructions carefully before using IZERVAY.
- •
- The IZERVAY kit includes a glass vial, filter needle, and an empty syringe. The glass vial, filter needle, and empty syringe are for single use only.
- •
- Store IZERVAY in the refrigerator at temperatures between 2ºC to 8ºC (36ºF to 46ºF). Do not freeze. Do not shake.
- •
- Prior to use, allow IZERVAY to reach room temperature, 20⁰C to 25⁰C (68⁰F to 77⁰F). The IZERVAY vial may be kept at room temperature for up to 24 hours. Keep the vial in the original carton to protect from light.
- •
- Use aseptic technique to carry out the preparation of the intravitreal injection.
- •
- Each vial should only be used for the treatment of a single eye.
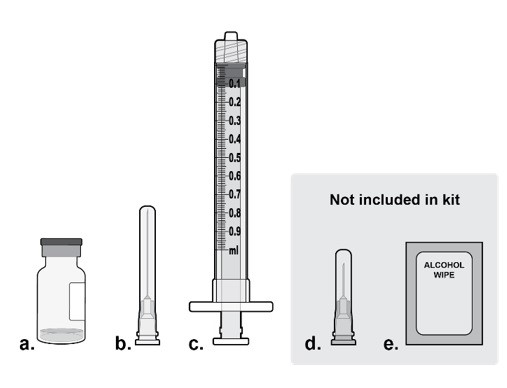
Step 1: Gather Supplies
Gather the following supplies (see Figure A):
- a.
- One IZERVAY vial (included)
- b.
- One sterile 5-micron filter needle 19-gauge x 1½ inch (included)
- c.
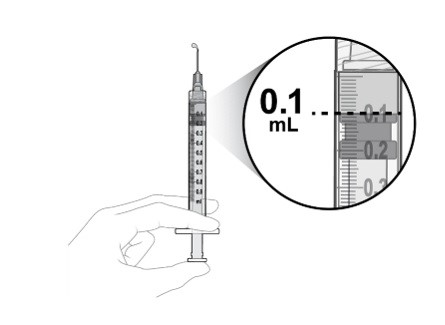
- One sterile 1 mL Luer lock syringe with a 0.1 mL dose mark (included)
- d.
- One sterile injection needle 30‑gauge x ½ inch (not included)
NOTE: a 30-gauge injection needle is recommended to avoid increased injection forces that could be experienced with smaller diameter needles.
- e.
- Alcohol swab (not included)
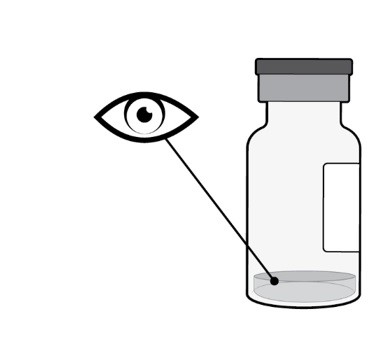
Step 2: Inspect Vial
Inspect the liquid in the vial. It should be a clear to slightly opalescent, colorless to slightly yellow liquid solution (see Figure B).
- Do not use if particulates, cloudiness, or discoloration are visible.
- Do not use if the packaging, vial, filter needle, injection needle, and/or empty syringe are expired, damaged, or have been tampered with.
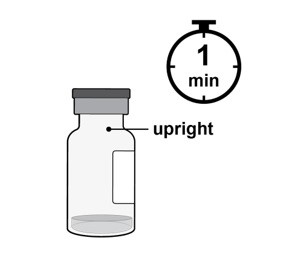
Step 3: Orient Vial
Place the vial upright on a flat surface for about 1 minute after removal from packaging to make sure all liquid settles at the bottom of the vial (see Figure C).
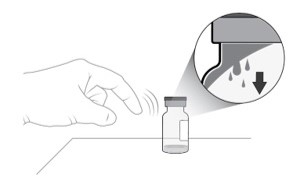
Gently tap the vial with your finger to remove any liquid that may stick to the top of the vial (see Figure D).
______________________________________
Step 4: Clean Vial
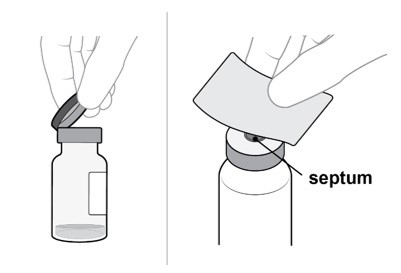
Remove the flip-off cap from the vial (see Figure E).
Gently wipe the vial septum with an alcohol swab (see Figure F).
Step 5: Attach Filter Needle
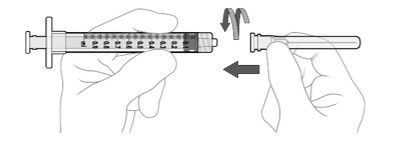
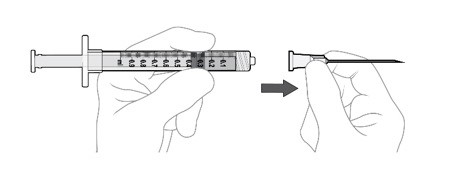
Using aseptic technique, firmly attach the included 19-gauge x 1½ inch filter needle onto the 1 mL Luer lock syringe and twist clockwise to secure (see Figure G).
Step 6: Insert Filter Needle into Vial
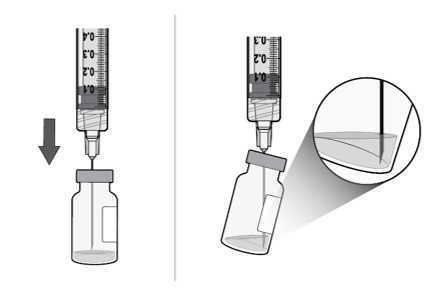
Using aseptic technique, push the filter needle all the way into the center of the vial septum (see Figure H).
Tilt the vial slightly so that the needle touches the bottom edge of the vial (see Figure I).
Rotate the filter needle so that the bevel is submerged into the liquid to avoid introduction of air.
Step 7: Withdraw Liquid
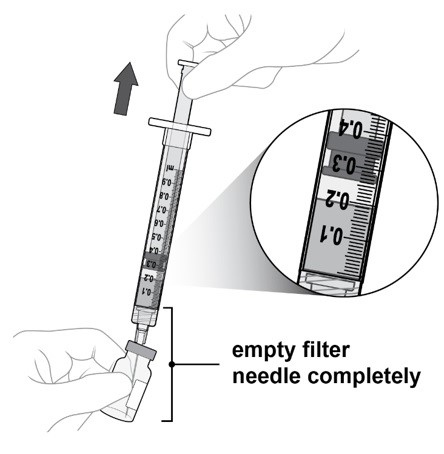
Slowly withdraw all the liquid from the vial (see Figure J).
Draw the plunger rod back far enough to completely empty the filter needle.
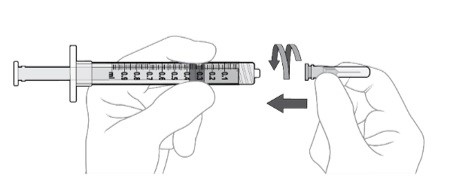
Step 8: Disconnect Filter Needle
Disconnect the filter needle from the syringe and dispose of it in accordance with local regulations (see Figure K).
Do not use the filter needle for the intravitreal injection.
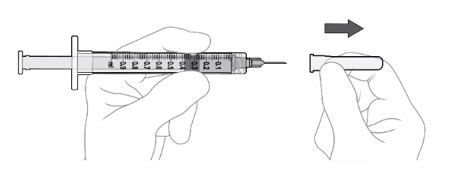
Step 9: Attach Injection Needle
Using aseptic technique, firmly attach the 30-gauge x ½ inch injection needle onto the Luer lock syringe. (see Figure L).
Carefully remove the plastic needle shield from the needle by pulling it straight off (see Figure M).
________________________________________
Step 10: Check Syringe
Check for air bubbles by holding the syringe with the needle pointing up. If there are any air bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure N).
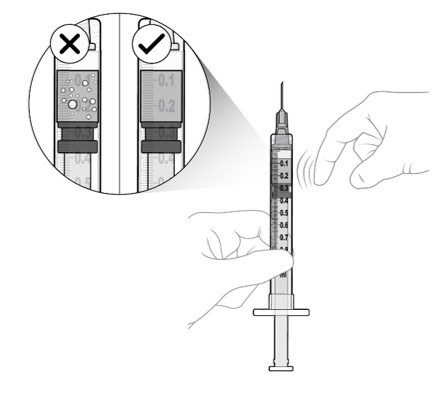
Step 11: Prepare Appropriate Dose
Slowly depress the plunger to:
- •
- Expel the air from the syringe
- •
- Align the rubber stopper tip to the 0.1 mL dose mark.
The syringe is now ready for the injection (see Figure O).
Make sure to give the injection immediately after preparing the dose.
2.4 Injection Procedure
Only 0.1 mL (2 mg) should be administered to deliver a single dose. Any excess volume should be disposed.
Prior to the intravitreal injection, patients should be monitored for elevated intraocular pressure (IOP) using tonometry [see Warnings and Precautions (5.3)]. If necessary, ocular hypotensive medication can be given to lower the IOP.
The intravitreal injection procedure must be carried out under controlled aseptic conditions, which includes the use of surgical hand disinfection, sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum topical microbicide should be given prior to the injection.
Inject slowly until the rubber stopper reaches the end of the syringe to deliver the volume of 0.1 mL. Confirm delivery of the full dose by checking that the rubber stopper has reached the end of the syringe barrel.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure (IOP). Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry.
Following intravitreal injection, patients should be instructed to report any symptoms suggestive of endophthalmitis (e.g., eye pain, redness of the eye, photophobia, blurring of vision) without delay [see Patient Counseling Information (17)].
Each vial and syringe should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new vial and syringe should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter needle, and injection needle should be changed before IZERVAY is administered to the other eye. Repeat the same procedure steps as above.
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Endophthalmitis and Retinal Detachments
Intravitreal injections may be associated with endophthalmitis and retinal detachments [see Adverse Reactions (6.1)]. Proper aseptic injection techniques must always be used when administering IZERVAY in order to minimize the risk of endophthalmitis [see Dosage and Administration (2.4)]. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay, to permit prompt and appropriate management [see Patient Counseling Information (17)].
5.2 Neovascular AMD
In clinical trials, use of IZERVAY was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (7% when administered monthly and 4% in the sham group) by Month 12. Patients receiving IZERVAY should be monitored for signs of neovascular AMD.
5.3 Increase in Intraocular Pressure
Transient increases in intraocular pressure (IOP) have been observed after an intravitreal injection, including with IZERVAY [see Adverse Reactions (6.1)]. Perfusion of the optic nerve head should be monitored following the injection and managed as needed [see Dosage and Administration (2.4)].
-
6 ADVERSE REACTIONS
The following potentially serious adverse reactions are described elsewhere in the labeling:
- •
- Ocular and periocular infections [see Contraindications (4.1)]
- •
- Active intraocular inflammation [see Contraindications (4.2)]
- •
- Endophthalmitis and retinal detachments [see Warnings and Precautions (5.1)]
- •
- Neovascular AMD [see Warnings and Precautions (5.2)]
- •
- Increase in intraocular pressure [see Warnings and Precautions (5.3)
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of avacincaptad pegol was evaluated in 733 patients with AMD in two sham-controlled studies (GATHER1 and GATHER2). Of these patients, 292 were treated with intravitreal IZERVAY 2 mg (0.1 mL of 20 mg/mL solution) [see Clinical Studies (14)]. Three hundred thirty-two (332) patients were assigned to sham.
Adverse reactions reported in ≥2% of patients who received treatment with IZERVAY pooled across GATHER1 and GATHER2, are listed below in Table 1.
Table 1: Common Ocular Adverse Reactions (≥2%) and greater than Sham in Study Eye Adverse Drug Reactions IZERVAY
N=292Sham
N=332- *
- Blurred vision includes visual impairment, vision blurred, visual acuity reduced, visual acuity reduced transiently
Conjunctival hemorrhage
13%
9%
Increased IOP
9%
1%
Blurred vision*
8%
5%
Choroidal neovascularization
7%
4%
Eye pain
4%
3%
Vitreous floaters
2%
<1%
Blepharitis
2%
<1%
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of IZERVAY administration in pregnant women. The use of IZERVAY may be considered following an assessment of the risks and benefits.
Administration of avacincaptad pegol to pregnant rats and rabbits throughout the period of organogenesis resulted in no evidence of adverse effects to the fetus or pregnant female at intravenous (IV) doses 5.1 times and 3.2 times the human exposure (based on AUC) at the maximum recommended human dose (MRHD) of 2 mg once monthly, respectively (see Data).
In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15%-20%, respectively.
Animal Data
An embryo fetal developmental toxicity study was conducted with pregnant rats. Pregnant rats received daily intravenous (IV) injections of avacincaptad pegol from day 6 to day 17 of gestation at 0.1, 0.4, 1.2 mg/kg/day. No maternal or embryofetal adverse effects were observed at any dose evaluated. An increase in the incidence of a non-adverse skeletal variation, described as short thoracolumbar (ossification site without distal cartilage) supernumerary ribs, was observed at all doses evaluated. The clinical relevance of this finding is unknown. Plasma exposures at the high dose were 5.1 times the MRHD, based on Area Under the Curve (AUC).
An embryo fetal developmental toxicity study was conducted with pregnant rabbits. Pregnant rabbits received daily IV injections of avacincaptad pegol from day 7 to day 19 of gestation at 0.12, 0.4, 1.2 mg/kg/day. No maternal or embryofetal adverse effects were observed at any dose evaluated. Plasma exposure in pregnant rabbits at the highest dose of 1.2 mg/kg/day was 3.2 times the human exposure at the MRHD, based on AUC.
8.2 Lactation
Risk Summary
There is no information regarding the presence of avacincaptad pegol in human milk, the effects of the drug on the breastfed infant, or the effects of avacincaptad pegol on milk production. Many drugs are transferred in human milk with the potential for absorption and adverse reactions in the breastfed child.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for IZERVAY, and any potential adverse effects on the breastfed infant from IZERVAY.
8.4 Pediatric Use
Safety and effectiveness of IZERVAY in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients who received IZERVAY in the two clinical trials, 90% (263/292) were ≥65 years and 61% (178/292) were ≥75 years of age. No significant differences in efficacy or safety of avacincaptad pegol were seen with increasing age in these studies. No dose adjustment is required in patients 65 years and above.
-
11 DESCRIPTION
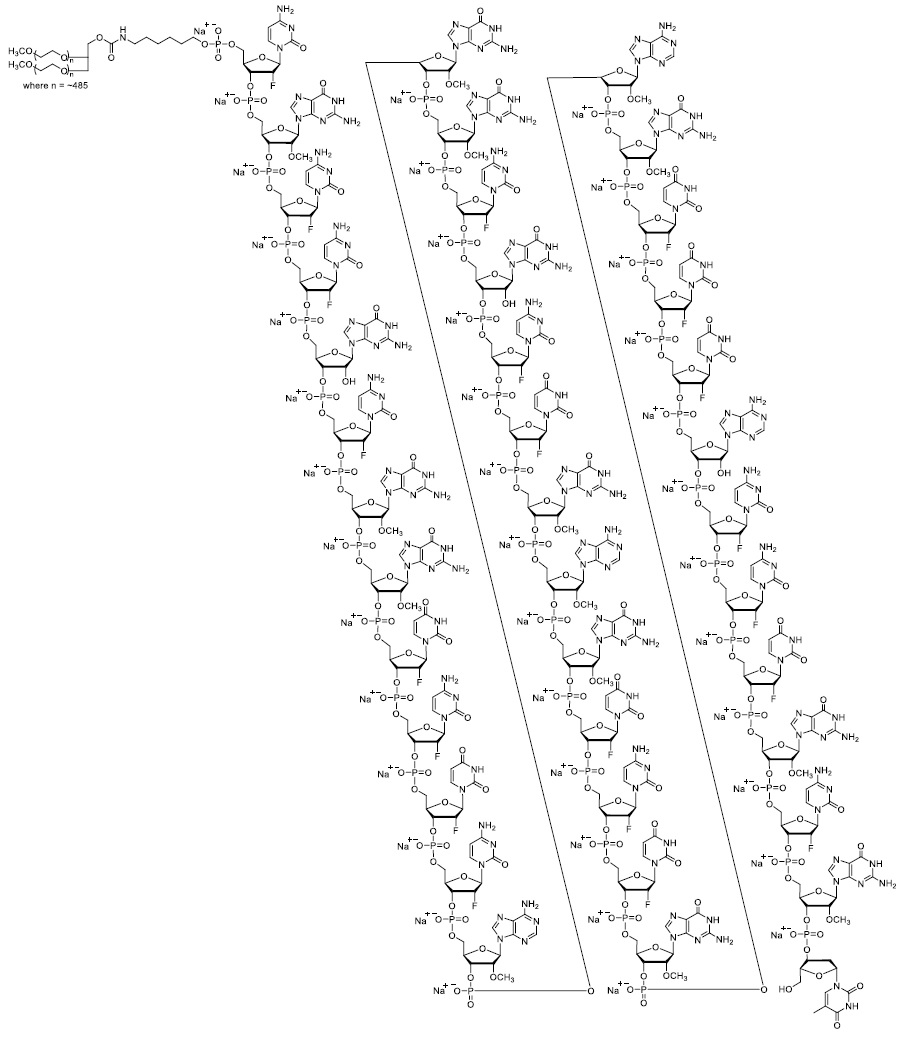
IZERVAY contains avacincaptad pegol sodium, a complement C5 inhibitor. Avacincaptad pegol is a ribonucleic acid (RNA) aptamer, covalently bound to an approximately 43-kiloDalton (kDa) branched polyethylene glycol (PEG) molecule.
The molecular formula of avacincaptad pegol (free acid form) is C395H492N142O262P39F21((CH2)2O)n where n~970 and the molecular weight is approximately 56 kDa. The structure of avacincaptad pegol sodium is presented below.
IZERVAY (avacincaptad pegol intravitreal solution) is a sterile, clear to slightly opalescent, colorless to slightly yellowish solution in a single-dose glass vial for intravitreal administration. Each single-dose vial is designed to deliver 0.1 mL of solution containing 2 mg avacincaptad pegol (oligonucleotide basis), 0.198 mg dibasic sodium phosphate heptahydrate, 0.0256 mg monobasic sodium phosphate monohydrate, and 0.83 mg sodium chloride. IZERVAY is formulated in Water for Injection, with a target pH of 7.3. IZERVAY does not contain an anti-microbial preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Avacincaptad pegol is an RNA aptamer, a PEGylated oligonucleotide that binds to and inhibits complement protein C5. By inhibiting C5, avacincaptad pegol may prevent its cleavage to C5a and C5b thus decreasing membrane attack complex (MAC) formation.
12.2 Pharmacodynamics
Increased GA area growth is reflective of loss of photoreceptors and AMD disease progression. Reductions in the rate GA growth were observed from baseline through the first year of treatment across avacincaptad pegol treatment groups in studies GATHER1 and GATHER2.
12.3 Pharmacokinetics
Absorption/Distribution
Following a single dose of avacincaptad pegol, maximum avacincaptad pegol plasma concentrations (Cmax) are estimated to occur approximately 7 days post-dose and mean (CV%) free avacincaptad pegol plasma Cmax is estimated to be 68.4 ng/mL (57.8%) in neovascular AMD (nAMD) patients. The AUC0-28 days following a single 2 mg dose is 1064 day∙ng/mL. Based on a population pharmacokinetic analysis of patients with nAMD, predicted steady state avacincaptad pegol Cmax is 83.9 ng/mL after monthly intravitreal administration of avacincaptad pegol 2 mg.
In humans, avacincaptad pegol plasma concentrations are predicted to be approximately 7,000-fold lower than vitreal concentrations.
Metabolism/Elimination
Metabolism and elimination of avacincaptad pegol has not been fully characterized. Avacincaptad pegol is expected to be catabolized by endonucleases and exonucleases to oligonucleotides of shorter lengths which may be excreted renally, in similar manner to the elimination of endogenous RNA. The estimated apparent systemic half-life of avacincaptad pegol is approximately 12 days.
Specific Populations
Following repeat monthly intravitreal dose administration of 2 mg avacincaptad pegol, no differences in the systemic pharmacokinetics of avacincaptad pegol were observed based on age, gender, and body weight. No special dosage modification is required for any of the populations that have been studied (e.g., age, gender, and body weight). The effect of severe renal impairment or any degree of hepatic impairment on the pharmacokinetics of avacincaptad pegol is unknown. As significant increases in plasma avacincaptad pegol exposures are not expected with intravitreal route of administration, no dosage adjustment is needed based on renal or hepatic impairment status.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
No studies have been conducted on the carcinogenic potential of avacincaptad pegol.
Mutagenesis
Avacincaptad pegol was negative in in vitro (bacterial reverse mutation assay, chromosomal aberration in mammalian cells) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
Studies to evaluate the effect of avacincaptad pegol on male or female fertility in animals have not been performed.
-
14 CLINICAL STUDIES
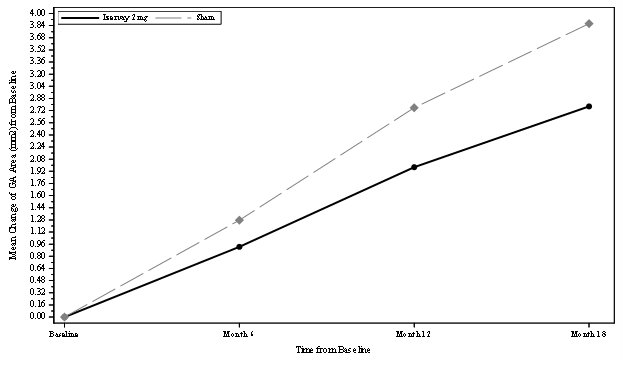
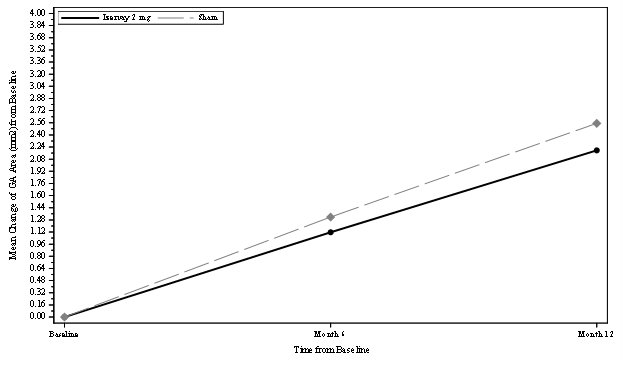
The safety and efficacy of IZERVAY were demonstrated in two randomized, multi-center, double-masked, sham-controlled, 18- and 12-month studies (GATHER1-NCT02686658 and GATHER2-NCT04435366, respectively) in patients with GA due to AMD. Patient ages ranged from 51 to 97 years with a mean of 77 years. In total, 292 patients were treated with avacincaptad pegol 2 mg, and 332 patients received sham.
In GATHER1 and GATHER2, the mean rate of GA growth (slope) from baseline to Month 12, measured by Fundus Autofluorescence (FAF) was evaluated at 3 time points: baseline, Month 6, and Month 12. Data are available through month 18 for GATHER1 and month 12 for GATHER2. At any time during the GATHER2 study, patients that developed choroidal neovascularization were concomitantly treated with anti-VEGF therapy.
In each study, over a 12-month period, there was a statistically significant reduction of the rate of GA growth (0.10 mm/year; p<0.01 in GATHER1 and 0.05 mm/year; p<0.01 in GATHER2 with square root transformed data) in patients treated with IZERVAY compared to sham. The observed results are shown in Table 2, Figure 1, and Figure 2 below.
Table 2: Efficacy Outcomes at Month 12 in GATHER1 and GATHER2 Studies Primary Efficacy Endpoint
(MMRM Analysis)
GATHER1
GATHER2
IZERVAY
N=67
Sham
N=110
IZERVAY
N=225
Sham
N=222
GA Rate of Growth (mm2/year) (observed)*
1.22
1.89
1.75
2.12
Difference (95% CI) (mm2/year)
Difference % †
p value
0.67 (0.21-1.13)
35%
<0.01
0.38 (0.12-0.63)
18%
<0.01
Treatment effects in all pre-specified subgroups (e.g., age, gender, baseline GA disc area) were consistent with the results in the overall population.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
IZERVAY (avacincaptad pegol intravitreal solution) is supplied as a sterile, clear to slightly opalescent, colorless to slightly yellowish 20 mg/mL solution in a single-dose glass vial. Each glass vial contains an overfill amount to allow administration of a single 0.1 mL dose of solution containing 2 mg of avacincaptad pegol (oligonucleotide basis). Each IZERVAY carton (NDC 82829-002-01) contains one glass vial, one sterile 5-micron transfer filter needle (19-gauge x 1½ inch, 1.1 mm x 40 mm), and one sterile 1 mL Luer lock syringe.
16.2 Storage and Handling
Store IZERVAY in the refrigerator between 2°C to 8°C (36°F to 46°F). Do not freeze. Do not shake. Keep the vial in the original carton to protect from light.
Prior to use, the unopened glass vial of IZERVAY may be kept at room temperature, 20°C to 25°C (68°F to 77°F), for up to 24 hours. Ensure that the injection is given immediately after preparation of the dose.
-
17 PATIENT COUNSELING INFORMATION
Advise patients that following IZERVAY administration, patients are at risk of developing neovascular AMD, endophthalmitis, elevated intraocular pressure and retinal detachments. If the eye becomes red, sensitive to light, painful, or if a patient develops a change in vision, instruct the patient to seek immediate care from an ophthalmologist [see Warnings and Precautions (5.1, 5.2)].
Patients may experience temporary visual disturbances and blurring after an intravitreal injection with IZERVAY and the associated eye examinations [see Adverse Reactions (6.1)]. Advise patients not to drive or use machinery until visual function has recovered sufficiently.
IZERVAY™ (avacincaptad pegol intravitreal solution)
Distributed by:
Astellas Pharma US, Inc.
Northbrook, IL 60062
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IZERVAY
avacincaptad pegol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82829-002 Route of Administration INTRAVITREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVACINCAPTAD PEGOL SODIUM (UNII: K86ENL12I5) (AVACINCAPTAD PEGOL - UNII:TT0V5JLG5B) AVACINCAPTAD PEGOL 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82829-002-01 1 in 1 CARTON 08/19/2023 1 0.35 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package 2 NDC:82829-002-99 1 in 1 CARTON 08/19/2023 2 0.35 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217225 08/19/2023 Labeler - Astellas Pharma US, Inc. (605764828)