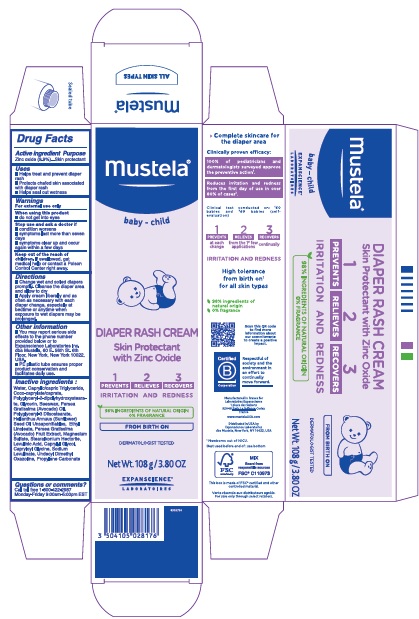
Label: MUSTELA DIAPER RASH 1 2 3- zinc oxide cream
- NDC Code(s): 64768-2922-1
- Packager: Expanscience Laboratories d/b/a Mustela
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, Caprylic/capric Triglyceride, Coco-caprylate/caprate, Polyglyceryl-2-dipolyhydroxystearate, Glycerin, Beeswax, Persea Gratissima (Avocado) Oil, Polyglyceryl-3 Diisostearate, Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables, Ethyl Linoleate, Persea Gratissima (Avocado) Fruit Extract, Magnesium Sulfate, Stearalkonium Hectorite, Levulinic Acid, Caprylyl Glycol, Capryloyl Glycine, Sodium Levulinate, Undecyl Dimethyl Oxazoline, Propylene Carbonate
- Questions or comments ?
- Company information
- Product Packaging
-
INGREDIENTS AND APPEARANCE
MUSTELA DIAPER RASH 1 2 3
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64768-2922 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 9.9 g in 100 g Inactive Ingredients Ingredient Name Strength SUNFLOWER OIL (UNII: 3W1JG795YI) LEVULINIC ACID (UNII: RYX5QG61EI) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) AVOCADO (UNII: SDS87L369F) AVOCADO OIL (UNII: 6VNO72PFC1) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SODIUM LEVULINATE (UNII: VK44E1MQU8) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) 4,4-DIMETHYLOXAZOLIDINE (UNII: 96J42P99ZA) WATER (UNII: 059QF0KO0R) YELLOW WAX (UNII: 2ZA36H0S2V) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) CAPRYLYL GLYCOL (UNII: 00YIU5438U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) ETHYL LINOLEATE (UNII: MJ2YTT4J8M) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64768-2922-1 1 in 1 CARTON 01/09/2017 1 107.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 01/09/2017 Labeler - Expanscience Laboratories d/b/a Mustela (181191057) Establishment Name Address ID/FEI Business Operations Laboratoires Expanscience d/b/a Mustela 347941502 manufacture(64768-2922)