Label: SENEGENCE MICROBIOME DEFENSE SUNSCREEN DAYTIME MOISTURIZER SPF15- zinc oxide, octisalate lotion
- NDC Code(s): 72644-638-01, 72644-638-02
- Packager: SGII, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
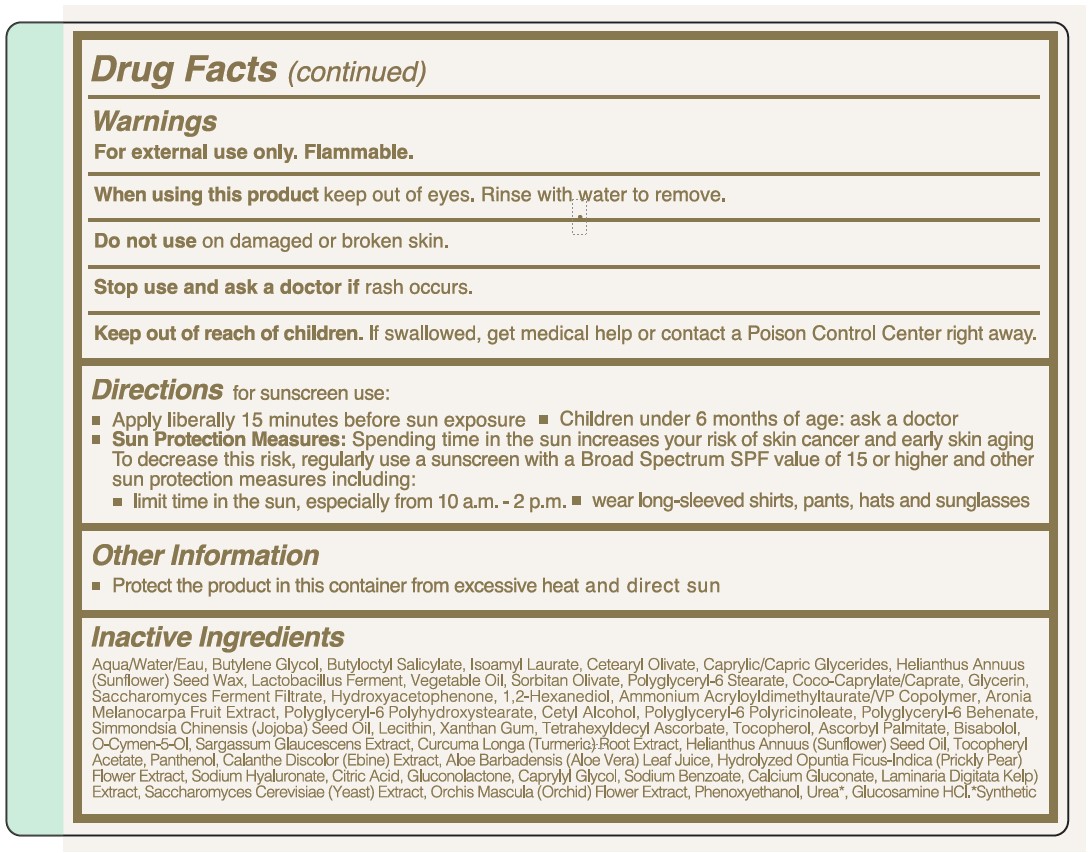
- Warnings
-
Directions
for sunscreen use:
- Apply liberally 15 minutes before sun exposure
- Children under 6 months of age: ask a doctor
- Sun Protection Measures: Spending time in the sun increases your skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Other Information
-
Inactive Ingredients
Aqua/Water/Eau, Butylene Glycol, Butyloctyl Salicylate, Isoamyl Laurate, Cetearyl Olivate, Caprylic/Capric Glycerides, Helianthus Annuus (Sunflower) Seed Wax, Lactobacillus Ferment, Vegetable Oil, Sorbitan Olivate, Polyglyceryl-6 Stearate, Coco-Caprylate/Caprate, Glycerin, Saccharomyces Ferment Filtrate, Hydroxyacetophenone, 1,2-Hexanediol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Aronia Melanocarpa Fruit Extract, Polyglyceryl-6 Polyhydroxystearate, Cetyl Alcohol, Polyglyceryl-6 Polyricinoleate, Polyglyceryl-6 Behenate, Simmondsia Chinensis (Jojoba) Seed Oil, Lecithin, Xanthan Gum, Tetrahexyldecyl AScorbate, Tocopherol, Ascorbyl Palmitate, Bisabolol, O-Cymen-5-Ol, Sargassum Glaucescens Extract, Curcuma Longa (Turmeric) Root Extract, Helianthus Annuus (Sunflower) Seed Oil, Tocopheryl Acetate, Panthenol, Calanthe Discolor (Ebine) Extract, Aloe Barbadensis (Aloe Vera) Leaf Juice, Hydrolyzed Opuntia Ficus-Indica (Prickly Pear) Flower Extract, Sodium Hyaluronate, Citric Acid, Gluconolactone, Caprylyl Glycol, Sodium Benzoate, Calcium Gluconate, Laminaria Digitata (Kelp) Extract, Saccharomyces Cerevisiae (Yeast) Extract, Orchis Mascula (Orchid) Flower Extract, Phenoxyethanol, Urea, Glucosamine HCl.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENEGENCE MICROBIOME DEFENSE SUNSCREEN DAYTIME MOISTURIZER SPF15
zinc oxide, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72644-638 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6 g in 100 mL Inactive Ingredients Ingredient Name Strength TURMERIC (UNII: 856YO1Z64F) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLUCONOLACTONE (UNII: WQ29KQ9POT) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALCIUM GLUCONATE (UNII: SQE6VB453K) ARONIA MELANOCARPA FRUIT (UNII: S935718Z2Q) TOCOPHEROL (UNII: R0ZB2556P8) ORCHIS MASCULA FLOWER (UNII: 6H1JQK35LA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) XANTHAN GUM (UNII: TTV12P4NEE) LEVOMENOL (UNII: 24WE03BX2T) SODIUM BENZOATE (UNII: OJ245FE5EU) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-6 STEARATE (UNII: ETY9Q81E2T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) SORBITAN OLIVATE (UNII: MDL271E3GR) ASCORBYL PALMITATE (UNII: QN83US2B0N) CETYL ALCOHOL (UNII: 936JST6JCN) JOJOBA OIL (UNII: 724GKU717M) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) O-CYMEN-5-OL (UNII: H41B6Q1I9L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) CETEARYL OLIVATE (UNII: 58B69Q84JO) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) LAMINARIA DIGITATA (UNII: 15E7C67EE8) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SUNFLOWER OIL (UNII: 3W1JG795YI) WATER (UNII: 059QF0KO0R) ISOAMYL LAURATE (UNII: M1SLX00M3M) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) UREA (UNII: 8W8T17847W) POLYGLYCERYL-6 BEHENATE (UNII: 4T2L7QI313) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) GLYCERIN (UNII: PDC6A3C0OX) PEG-5 CAPRYLIC/CAPRIC GLYCERIDES (UNII: S0SPK283TZ) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) PANTHENOL (UNII: WV9CM0O67Z) OPUNTIA FICUS-INDICA FLOWER (UNII: 83YSP51SMA) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72644-638-01 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/10/2024 2 NDC:72644-638-02 2 mL in 1 PACKET; Type 0: Not a Combination Product 05/09/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/10/2024 Labeler - SGII, INC (070096792) Establishment Name Address ID/FEI Business Operations TCI Co.,Ltd.-BioCosme PABP BRANCH 657732201 manufacture(72644-638)