Label: CHILDRENS ROBITUSSIN COUGH AND CHEST CONGESTION DM- dextromethorphan hydrobromide and guaifenesin liquid
- NDC Code(s): 0031-8702-13
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 8, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
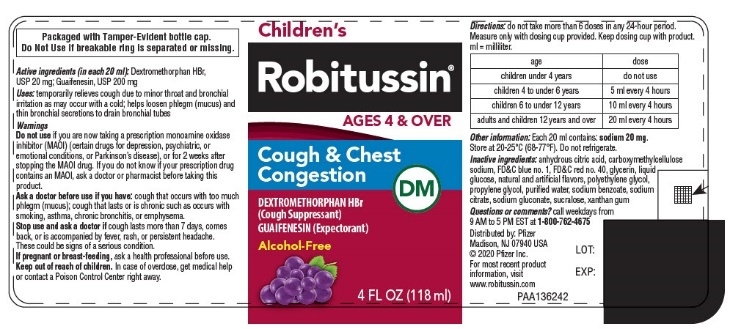
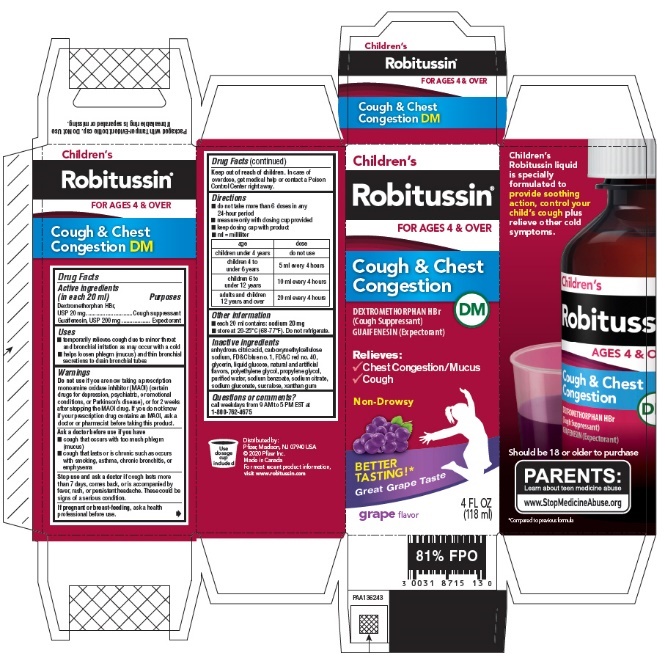
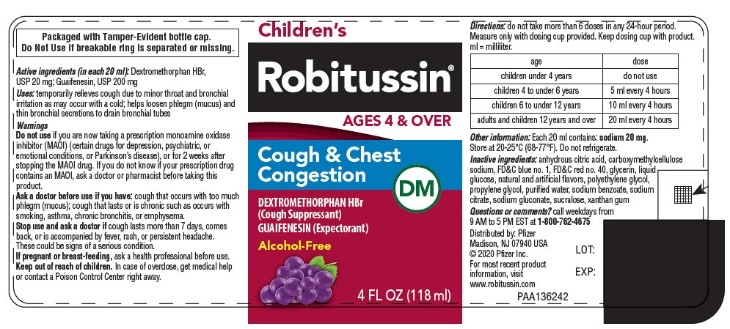
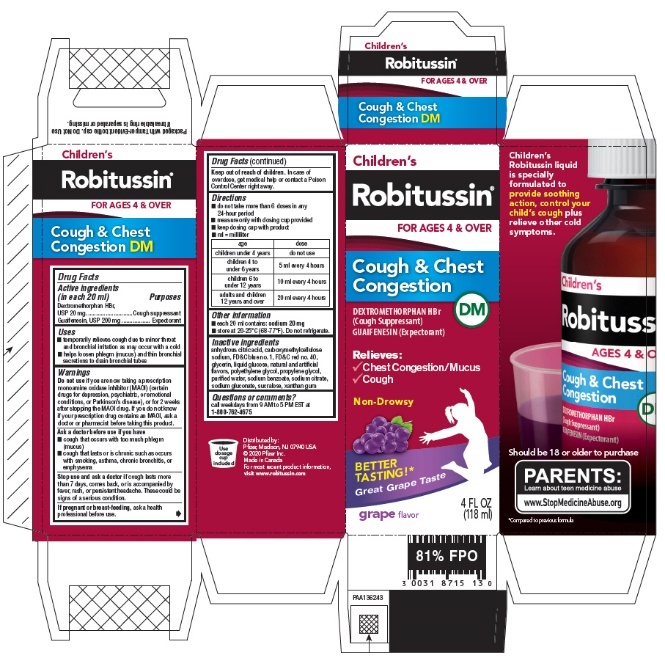
- Active ingredients (in each 20 ml)
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
-
DOSAGE & ADMINISTRATION
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- ml = milliliter
age dose children under 4 years
do not use
children 4 to under 6 years
5 ml every 4 hours
children 6 to under 12 years
10 ml every 4 hours
adults and children 12 years and over
20 ml every 4 hours
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118 ml Bottle Label
- PRINCIPAL DISPLAY PANEL - 118 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
CHILDRENS ROBITUSSIN COUGH AND CHEST CONGESTION DM
dextromethorphan hydrobromide and guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8702 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM GLUCONATE (UNII: R6Q3791S76) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PURPLE (purple) Score Shape Size Flavor GRAPE (grape) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8702-13 1 in 1 CARTON 05/12/2020 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/12/2020 Labeler - Haleon US Holdings LLC (079944263) Establishment Name Address ID/FEI Business Operations Pf Soins De Santé Sri 203812479 ANALYSIS(0031-8702) , LABEL(0031-8702) , MANUFACTURE(0031-8702) , PACK(0031-8702)