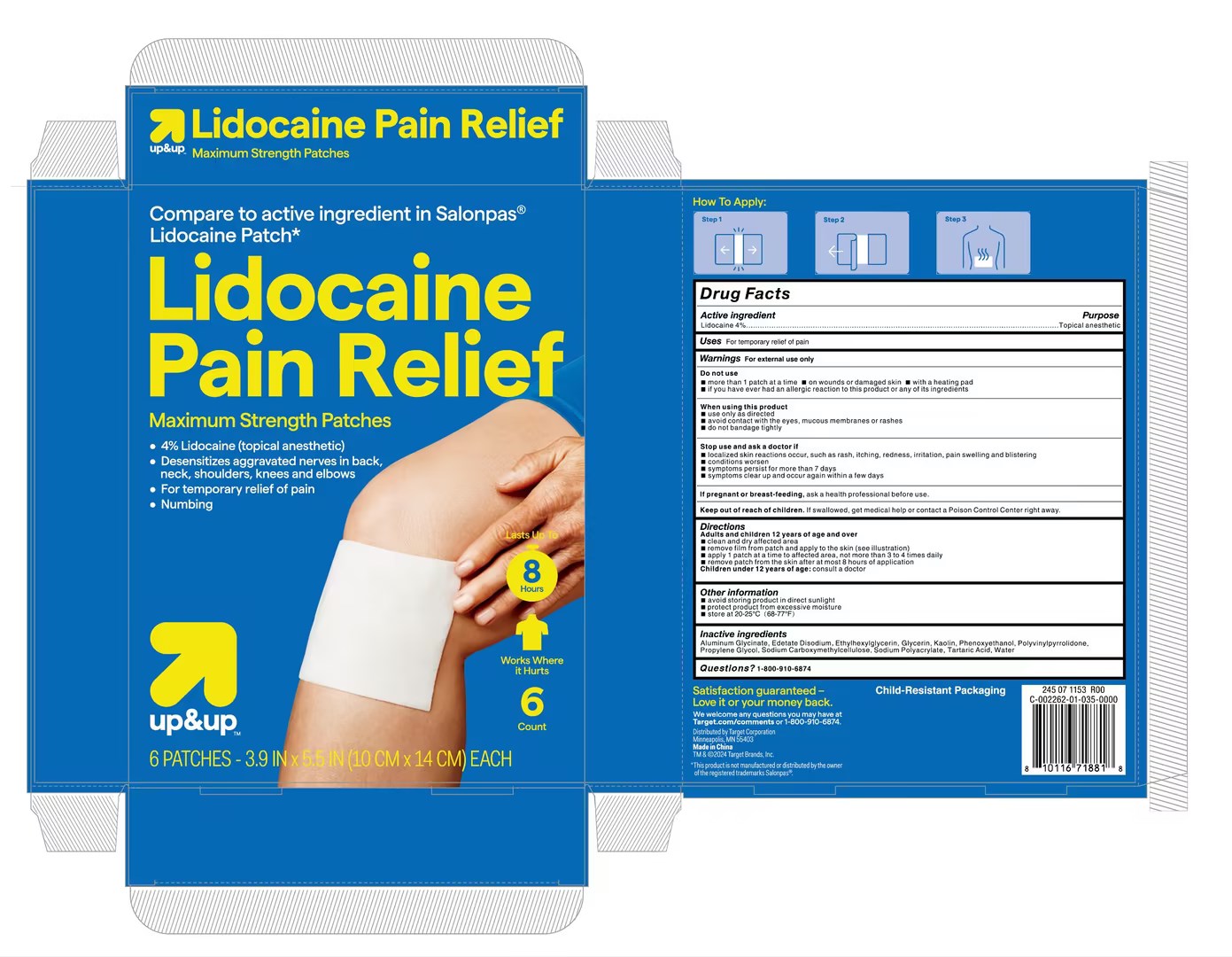
Label: LIDOCAINE PAIN RELIEF PATCH patch
- NDC Code(s): 83559-004-01
- Packager: Henan Enokon Medical Instrument Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Out Of Reach Of Children
-
Directions
Adults and children 12 years of age and over
clean and dry affected area
remove film from patch and apply to the skin (see illustration)
apply 1 patch at a time to affected area, not more than 3 to 4 times daily
remove patch from the skin after at most 8 hours of application
Children under 12 years of age: consult a doctor - Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE PAIN RELIEF PATCH
lidocaine pain relief patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83559-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) TARTARIC ACID (UNII: W4888I119H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) PHENOXYETHANOL (UNII: HIE492ZZ3T) POVIDONE (UNII: FZ989GH94E) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM ACRYLATE (UNII: 7C98FKB43H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83559-004-01 6 in 1 BOX; Type 0: Not a Combination Product 04/07/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/07/2024 Labeler - Henan Enokon Medical Instrument Co., Ltd. (701730676) Establishment Name Address ID/FEI Business Operations Henan Enokon Medical Instrument Co., Ltd. 701730676 manufacture(83559-004)