Label: MEPHYTON- phytonadione tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 50090-1753-0 - Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 0187-1704
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
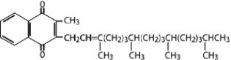
Phytonadione is a vitamin which is a clear, yellow to amber, viscous, and nearly odorless liquid. It is insoluble in water, soluble in chloroform and slightly soluble in ethanol. It has a molecular weight of 450.7.
Phytonadione is 2-methyl-3-phytyl-1, 4-naphthoquinone. Its empirical formula is C31H46O2 and its structural formula is:
Mephyton® (phytonadione) tablets containing 5 mg of phytonadione are yellow, compressed tablets, scored on one side. Inactive ingredients are acacia, calcium phosphate, colloidal silicon dioxide, lactose, magnesium stearate, starch, and talc.
-
CLINICAL PHARMACOLOGY
Mephyton tablets possess the same type and degree of activity as does naturally-occurring vitamin K, which is necessary for the production via the liver of active prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X). The prothrombin test is sensitive to the levels of three of these four factors – II, VII, and X. Vitamin K is an essential cofactor for a microsomal enzyme that catalyzes the posttranslational carboxylation of multiple, specific, peptide-bound glutamic acid residues in inactive hepatic precursors of factors II, VII, IX, and X. The resulting gamma-carboxyglutamic acid residues convert the precursors into active coagulation factors that are subsequently secreted by liver cells into the blood.
Oral phytonadione is adequately absorbed from the gastrointestinal tract only if bile salts are present. After absorption, phytonadione is initially concentrated in the liver, but the concentration declines rapidly. Very little vitamin K accumulates in tissues. Little is known about the metabolic fate of vitamin K. Almost no free unmetabolized vitamin K appears in bile or urine.
In normal animals and humans, phytonadione is virtually devoid of pharmacodynamic activity. However, in animals and humans deficient in vitamin K, the pharmacological action of vitamin K is related to its normal physiological function, that is, to promote the hepatic biosynthesis of vitamin K-dependent clotting factors.
Mephyton tablets generally exert their effect within 6 to 10 hours.
-
INDICATIONS AND USAGE
Mephyton is indicated in the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity.
Mephyton tablets are indicated in:
- •
- anticoagulant-induced prothrombin deficiency caused by coumarin or indanedione derivatives;
- •
- hypoprothrombinemia secondary to antibacterial therapy;
- •
- hypoprothrombinemia secondary to administration of salicylates;
- •
- hypoprothrombinemia secondary to obstructive jaundice or biliary fistulas but only if bile salts are administered concurrently, since otherwise the oral vitamin K will not be absorbed.
- CONTRAINDICATIONS
-
WARNINGS
An immediate coagulant effect should not be expected after administration of phytonadione.
Phytonadione will not counteract the anticoagulant action of heparin.
When vitamin K1 is used to correct excessive anticoagulant-induced hypoprothrombinemia, anticoagulant therapy still being indicated, the patient is again faced with the clotting hazards existing prior to starting the anticoagulant therapy.
Phytonadione is not a clotting agent, but overzealous therapy with vitamin K1 may restore conditions which originally permitted thromboembolic phenomena. Dosage should be kept as low as possible, and prothrombin time should be checked regularly as clinical conditions indicate.
Repeated large doses of vitamin K are not warranted in liver disease if the response to initial use of the vitamin is unsatisfactory. Failure to respond to vitamin K may indicate a congenital coagulation defect or that the condition being treated is unresponsive to vitamin K.
-
PRECAUTIONS
General
Vitamin K1 is fairly rapidly degraded by light; therefore, always protect Mephyton from light. Store Mephyton in closed original carton until contents have been used (see HOW SUPPLIED, Storage).
Drug Interactions
Temporary resistance to prothrombin-depressing anticoagulants may result, especially when larger doses of phytonadione are used. If relatively large doses have been employed, it may be necessary when reinstituting anticoagulant therapy to use somewhat larger doses of the prothrombin-depressing anticoagulant or to use one which acts on a different principle, such as heparin sodium.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of carcinogenicity or impairment of fertility have not been performed with Mephyton. Mephyton at concentrations up to 2,000 mcg/plate, with or without metabolic activation, was negative in the Ames microbial mutagen test.
Pregnancy
Animal reproduction studies have not been conducted with Mephyton. It is also not known whether Mephyton can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Mephyton should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established with Mephyton. Hemolysis, jaundice, and hyperbilirubinemia in newborns, particularly in premature infants, have been reported with vitamin K.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Mephyton is administered to a nursing woman.
Geriatric Use
Clinical studies of Mephyton did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Severe hypersensitivity reactions, including anaphylactoid reactions and deaths, have been reported following parenteral administration. The majority of these reported events occurred following intravenous administration.
Transient “flushing sensations” and “peculiar” sensations of taste have been observed with parenteral phytonadione, as well as rare instances of dizziness, rapid and weak pulse, profuse sweating, brief hypotension, dyspnea, and cyanosis.
Hyperbilirubinemia has been observed in the newborn following administration of parenteral phytonadione. This has occurred rarely and primarily with doses above those recommended.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Mephyton Tablets Summary of Dosage Guidelines (See package insert for details.) Adults Initial Dosage Anticoagulant-Induced Prothrombin Deficiency
(caused by coumarin or indanedione derivatives)2.5 mg to 10 mg or up to 25 mg
(rarely 50 mg)Hypoprothrombinemia
Due to Other Causes
(antibiotics; salicylates or other drugs; factors limiting absorption or synthesis)2.5 mg to 25 mg or more (rarely up to 50 mg)
Anticoagulant-Induced Prothrombin Deficiency in Adults
To correct excessively prolonged prothrombin times caused by oral anticoagulant therapy, 2.5 to 10 mg or up to 25 mg initially is recommended. In rare instances 50 mg may be required. Frequency and amount of subsequent doses should be determined by prothrombin time response or clinical condition (see WARNINGS). If, in 12 to 48 hours after oral administration, the prothrombin time has not been shortened satisfactorily, the dose should be repeated.
Hypoprothrombinemia Due to Other Causes in Adults
If possible, discontinuation or reduction of the dosage of drugs interfering with coagulation mechanisms (such as salicylates, antibiotics) is suggested as an alternative to administering concurrent Mephyton. The severity of the coagulation disorder should determine whether the immediate administration of Mephyton is required in addition to discontinuation or reduction of interfering drugs.
A dosage of 2.5 to 25 mg or more (rarely up to 50 mg) is recommended, the amount and route of administration depending upon the severity of the condition and response obtained.
The oral route should be avoided when the clinical disorder would prevent proper absorption. Bile salts must be given with the tablets when the endogenous supply of bile to the gastrointestinal tract is deficient.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- phytonadione
-
INGREDIENTS AND APPEARANCE
MEPHYTON
phytonadione tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50090-1753(NDC:0187-1704) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 5 mg Inactive Ingredients Ingredient Name Strength Acacia (UNII: 5C5403N26O) Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium stearate (UNII: 70097M6I30) Starch, Corn (UNII: O8232NY3SJ) Talc (UNII: 7SEV7J4R1U) CALCIUM PHOSPHATE, UNSPECIFIED FORM (UNII: 97Z1WI3NDX) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color YELLOW (pale yellow) Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code VRX;405;Mephyton Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50090-1753-0 5 in 1 BOTTLE; Type 0: Not a Combination Product 03/19/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA010104 03/19/2013 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-1753) , REPACK(50090-1753)