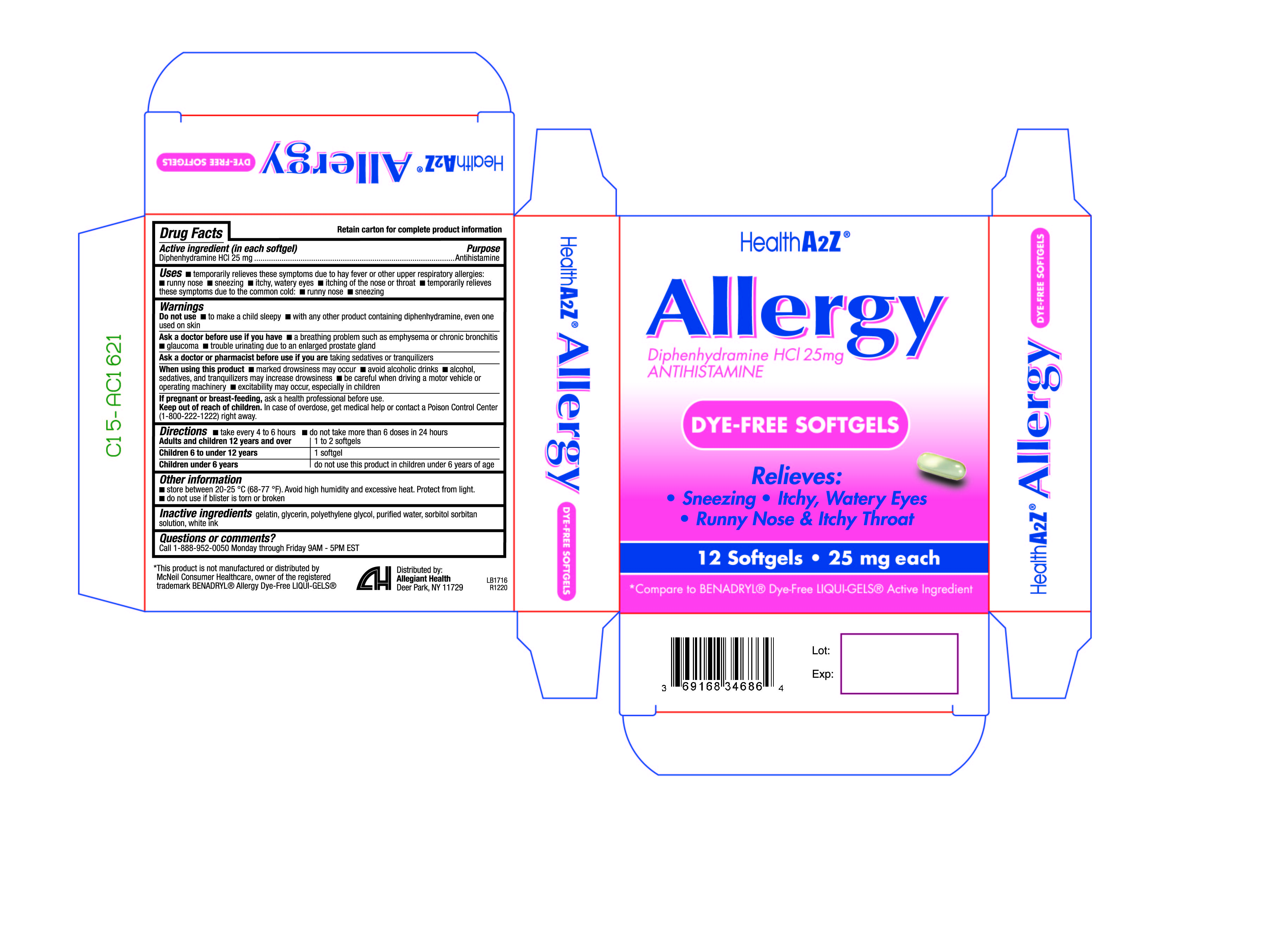
Label: ALLERGY DYEFREE capsule, liquid filled
- NDC Code(s): 69168-409-86
- Packager: Allegiant Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 26, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
- Purpose
- Use(s)
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- Storage
- Inactive ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALLERGY DYEFREE
allergy dyefree capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69168-409 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SORBITOL SOLUTION (UNII: 8KW3E207O2) Product Characteristics Color yellow (Clear) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code PC4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69168-409-86 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/27/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 09/15/2013 Labeler - Allegiant Health (079501930)