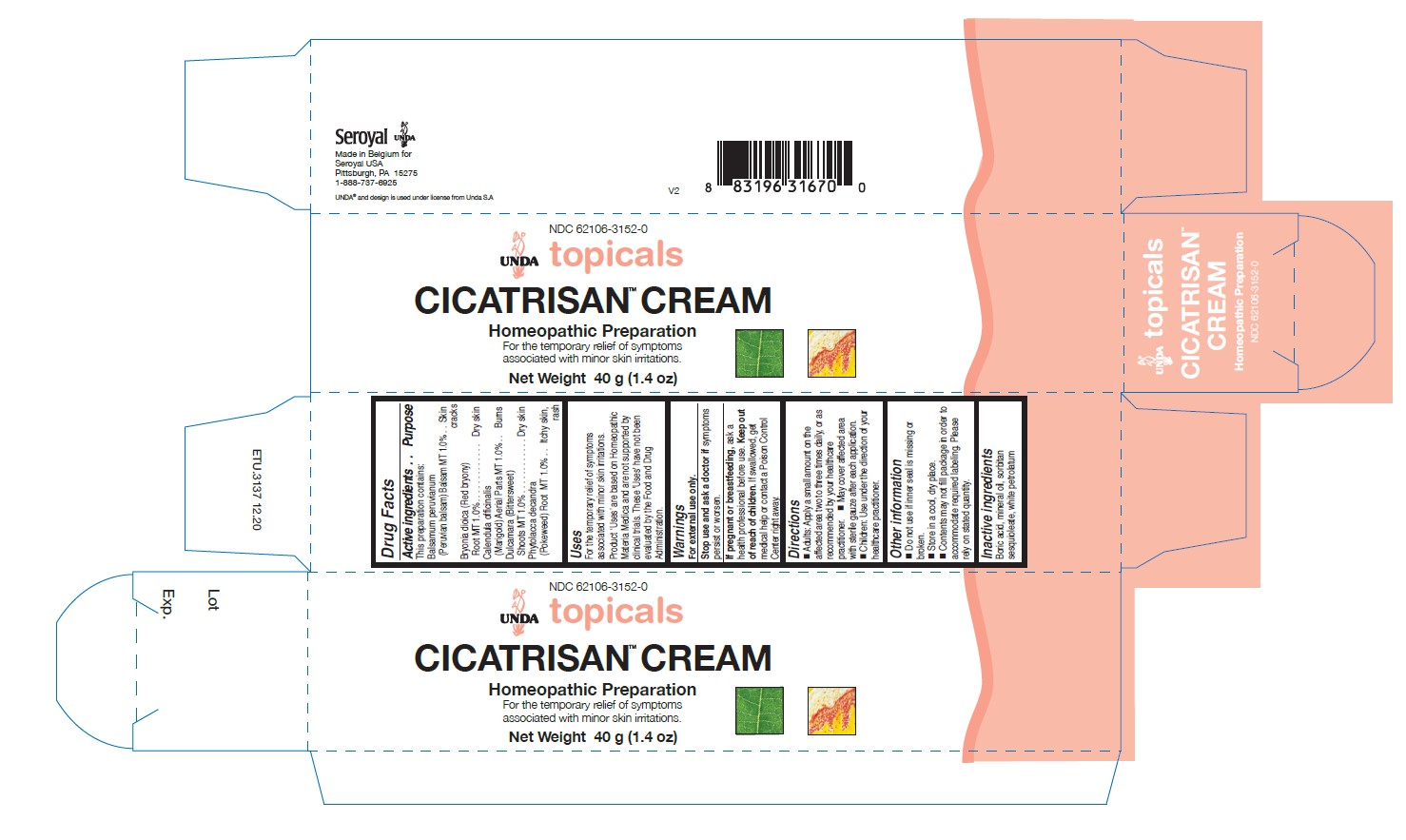
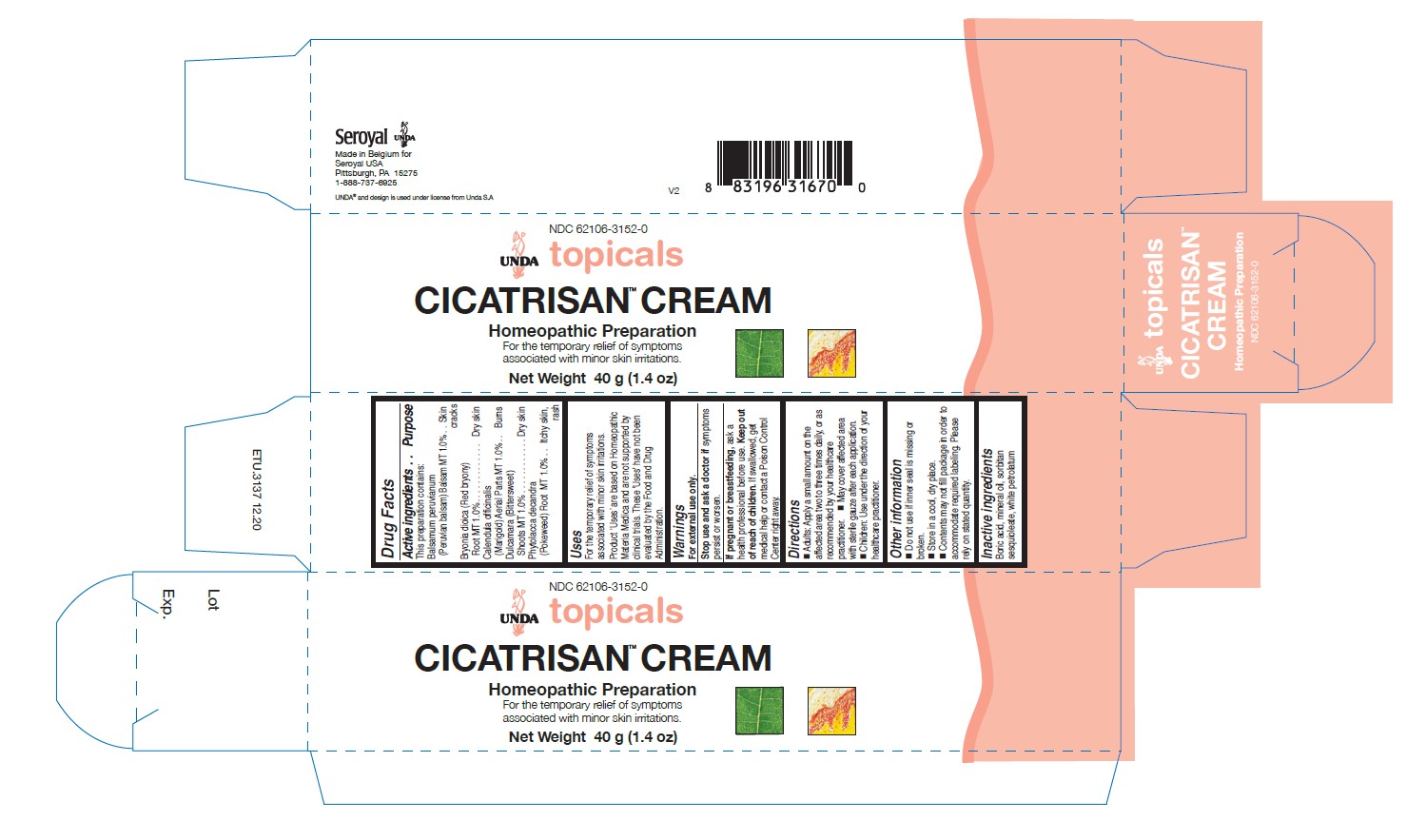
Label: CICATRISAN CREAM- calendula officinalis, phytolacca decandra, bryonia dioica, balsamum peruvianum, dulcamara cream
- NDC Code(s): 62106-3152-0
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms
associated with minor skin irritations.Directions
Adults: Apply a small amount on the
affected area two to three times daily, or as
recommended by your healthcare
practitioner. May cover affected area with
sterile gauze after each application.
Children: Use under the direction of your
healthcare practitioner. - OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CICATRISAN CREAM
calendula officinalis, phytolacca decandra, bryonia dioica, balsamum peruvianum, dulcamara creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-3152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BALSAM PERU (UNII: 8P5F881OCY) (BALSAM PERU - UNII:8P5F881OCY) BALSAM PERU 1 [hp_X] in 40 g BRYONIA DIOICA ROOT (UNII: 53UB5FH7CX) (BRYONIA DIOICA ROOT - UNII:53UB5FH7CX) BRYONIA DIOICA ROOT 1 [hp_X] in 40 g SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 1 [hp_X] in 40 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 40 g PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 1 [hp_X] in 40 g Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) PARAFFIN (UNII: I9O0E3H2ZE) MINERAL OIL (UNII: T5L8T28FGP) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-3152-0 1 in 1 CARTON 05/07/2015 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/07/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-3152)