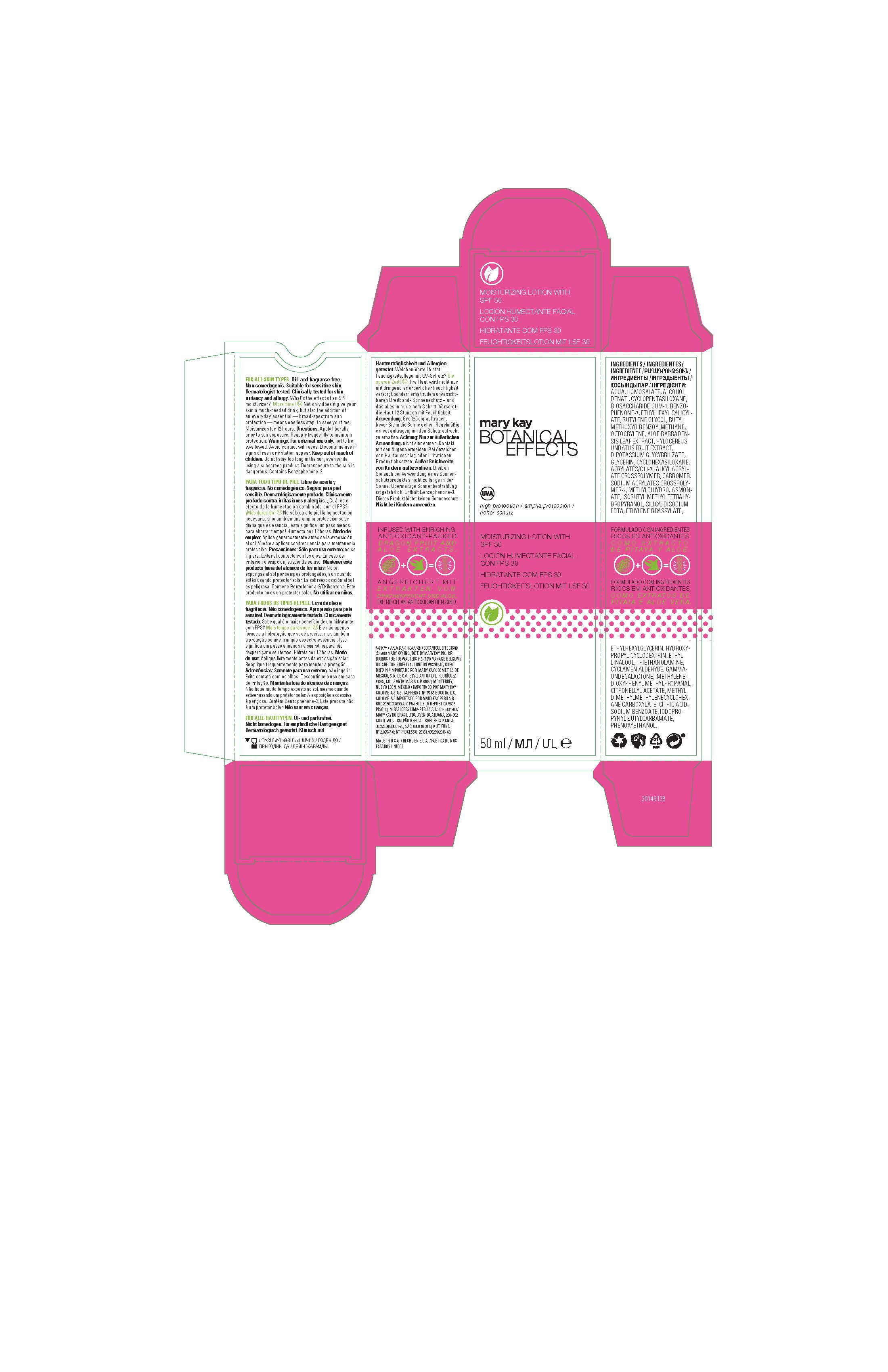
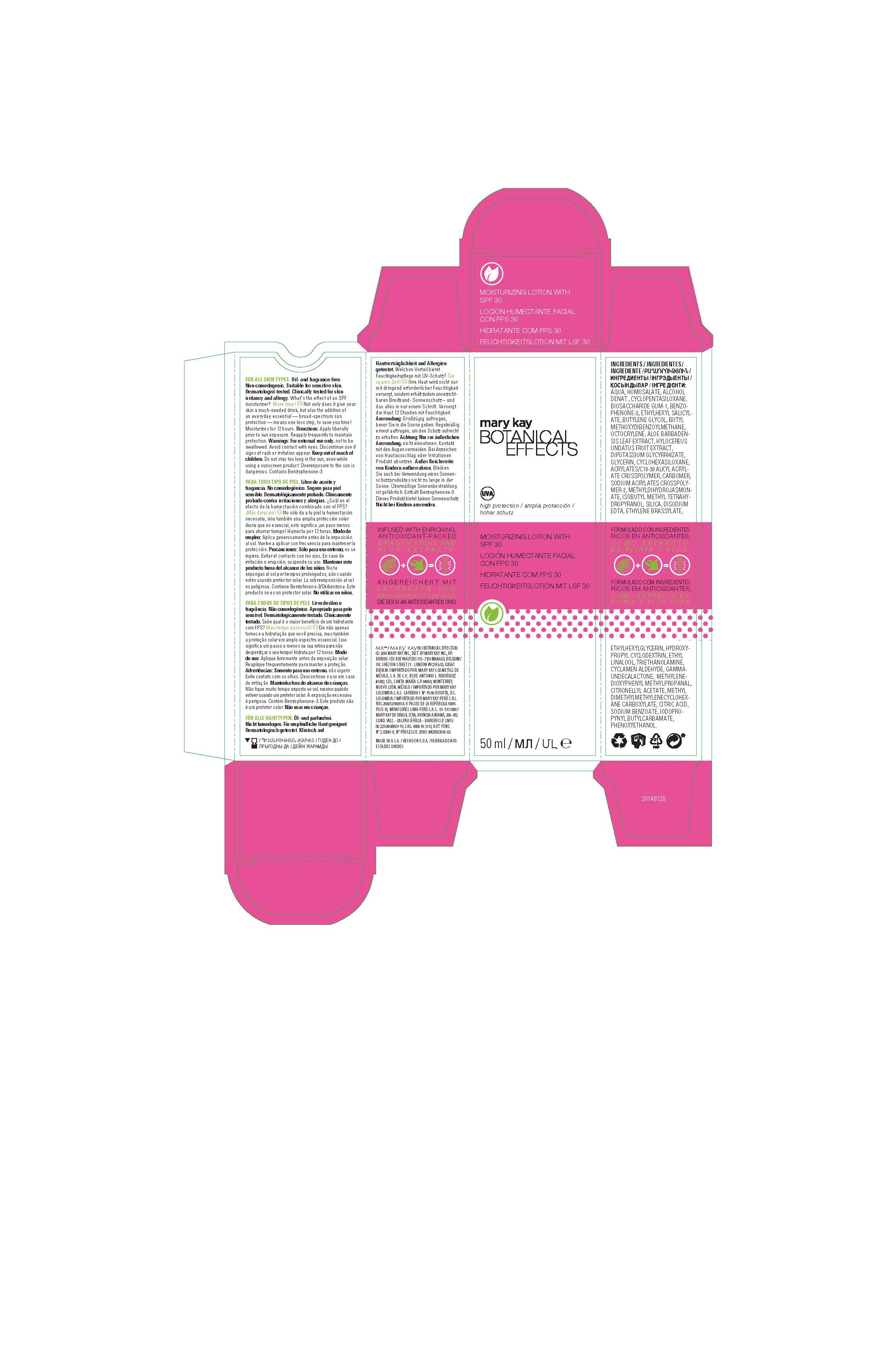
Label: BOTANICAL EFFECTS MOISTURIZER SPF 30- avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotion
- NDC Code(s): 51531-4850-7
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated August 24, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- Warnings:
- Directions:
-
Ingredients:
AQUA, HOMOSALATE, ALCOHOL DENAT., CYCLOPENTASILOXANE, BIOSACCHARIDE GUM-1, BENZOPHENONE-3, ETHYLHEXYL SALICYLATE, BUTYLENE GLYCOL, BUTYL METHOXYDIBENZOYLMETHANE, OCTOCRYLENE, ALOE BARBADENSIS LEAF EXTRACT, HYLOCEREUS UNDATUS FRUIT EXTRACT, DIPOTASSIUM GLYCYRRHIZATE, GLYCERIN, CYCLOHEXASILOXANE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, CARBOMER, SODIUM ACRYLATES CROSSPOLYMER-2, METHYLDIHYDROJASMONATE, ISOBUTYL METHYL TETRAHYDROPYRANOL, SILICA, DISODIUM EDTA, ETHYLENE BRASSYLATE, ETHYLHEXYLGLYCERIN, HYDROXYPROPYL CYCLODEXTRIN, ETHYL LINALOOL, TRIETHANOLAMINE, CYCLAMEN ALDEHYDE, GAMMA-UNDECALACTONE, METHYLENEDIOXYPHENYL METHYLPROPANAL, CITRONELLYL ACETATE, METHYL DIMETHYLMETHYLENECYCLOHEXANE CARBOXYLATE, CITRIC ACID, SODIUM BENZOATE, IODOPROPYNYL BUTYLCARBAMATE, PHENOXYETHANOL.
- Principal Display Panel - 50 mL carton
-
INGREDIENTS AND APPEARANCE
BOTANICAL EFFECTS MOISTURIZER SPF 30
avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-4850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.75 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4.5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) DENATONIUM BENZOATE ANHYDROUS (UNII: M5BA6GAF1O) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) GLYCERIN (UNII: PDC6A3C0OX) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) HYLOCEREUS UNDATUS FRUIT (UNII: WUG58TD53X) 2-ISOBUTYL-4-METHYLTETRAHYDROPYRAN-4-OL (UNII: VK5ZHH2T3F) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYDROXYPROPYL .ALPHA.-CYCLODEXTRIN (UNII: ZFR0T80O4Y) ETHYL LINALOOL (UNII: SF2JS9GF5T) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) TROLAMINE (UNII: 9O3K93S3TK) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLAMEN ALDEHYDE (UNII: 4U37UX0E1E) .GAMMA.-UNDECALACTONE (UNII: QB1T0AG2YL) 3-(3,4-METHYLENEDIOXYPHENYL)-2-METHYLPROPANAL (UNII: L65EG8H6PA) CITRONELLYL ACETATE (UNII: IZ420RT3OY) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-4850-7 1 in 1 CARTON 08/21/2014 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/21/2014 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-4850)