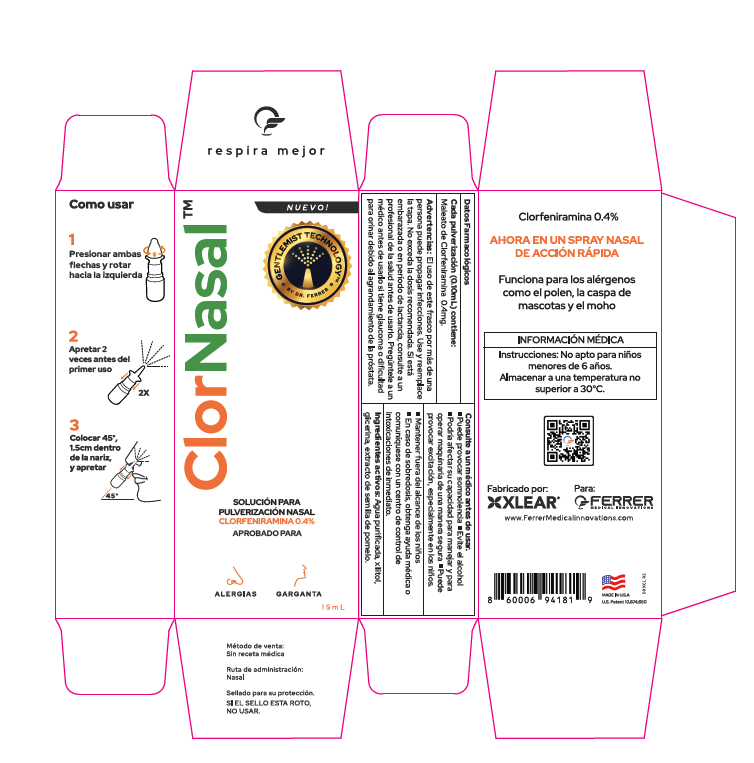
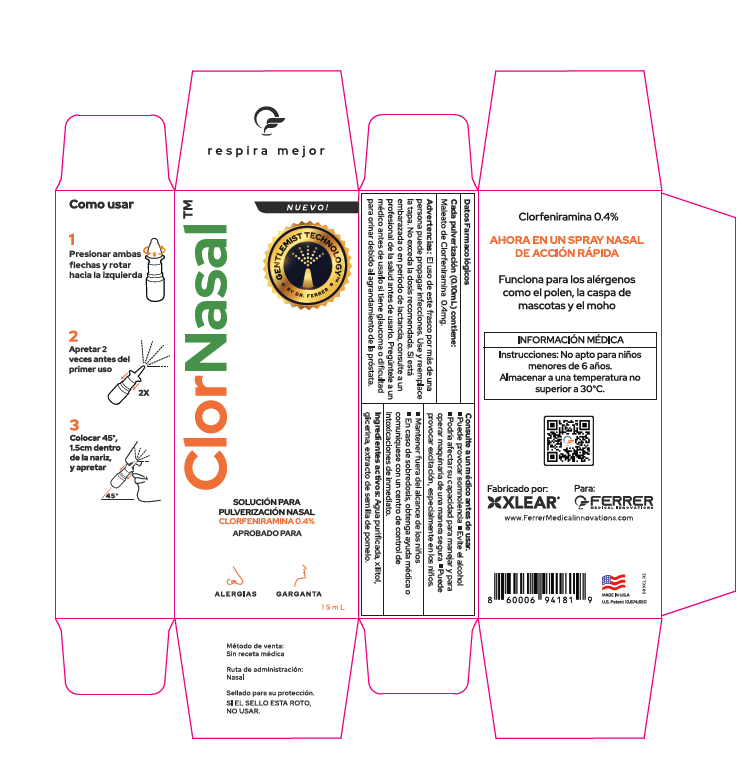
Label: CLORNASAL- chlorpheniramine maleate nasal spray spray, metered
- NDC Code(s): 82643-103-15
- Packager: FERRER MEDICAL INNOVATIONS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLORNASAL
chlorpheniramine maleate nasal spray spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82643-103 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM BICARBONATE (UNII: 8MDF5V39QO) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) XYLITOL (UNII: VCQ006KQ1E) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82643-103-15 1 in 1 CARTON 05/01/2024 1 15 mL in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/01/2024 Labeler - FERRER MEDICAL INNOVATIONS LLC (041608434) Registrant - FERRER MEDICAL INNOVATIONS LLC (041608434) Establishment Name Address ID/FEI Business Operations Xlear Inc. 839884058 manufacture(82643-103)