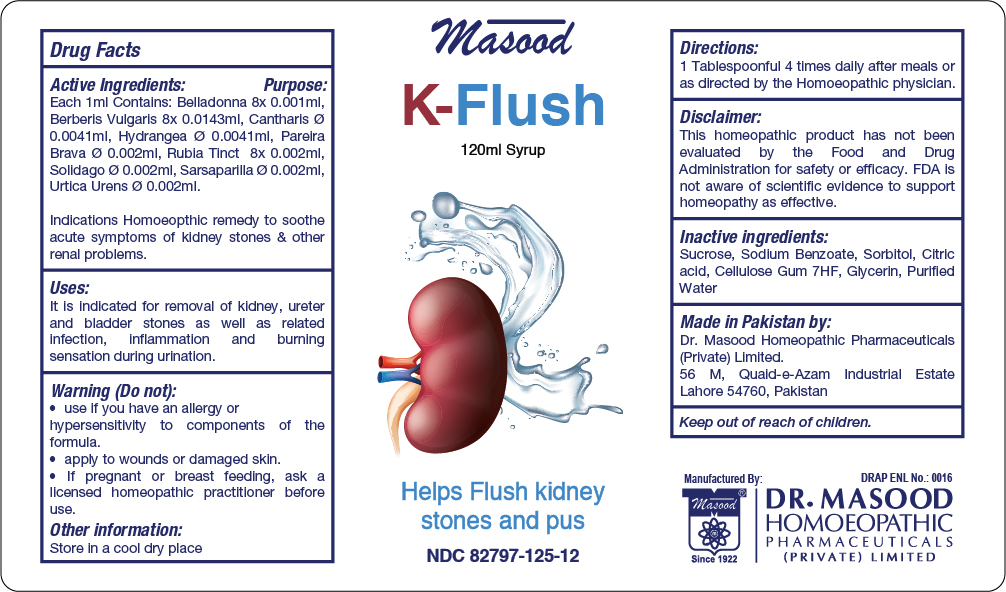
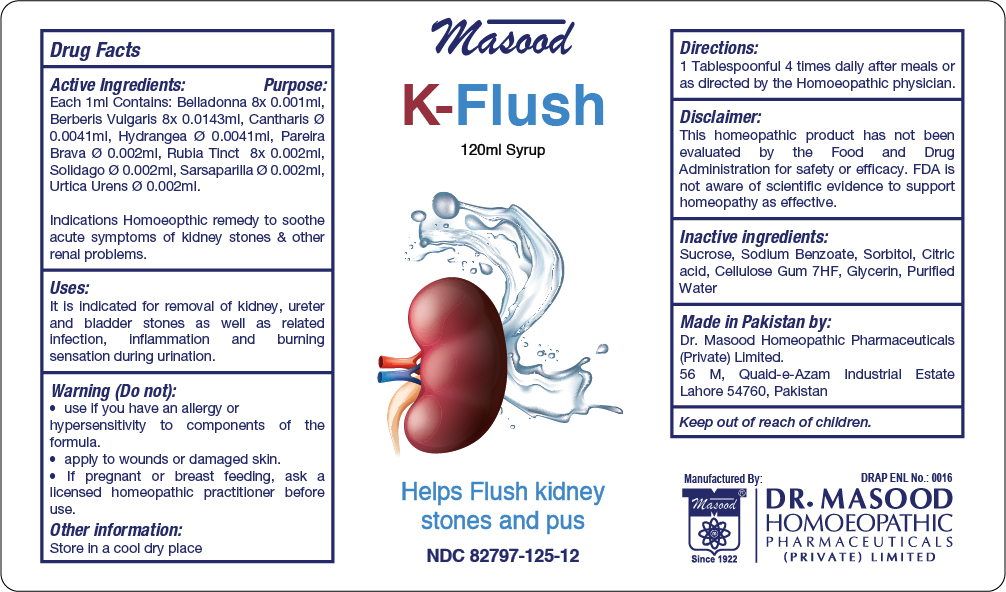
Label: K FLUSH- belladonna, berberls vulgarls, cantharis, , hydrangea, parelra brava, rubla tinct, solidago, sarsaparilla, urtica urens syrup
- NDC Code(s): 82797-125-12
- Packager: Dr. Masood Homeopathic Pharmaceuticals Private Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
-
PURPOSE
Active Ingredients Purpose
Belladonna 8x Cystitis, burning pain in urinary tract. Acute Urinary Infections. Spasmodic pains along ureter
Berberls Vulgarls Used for Renal troubles
Cantharis Helps control frequency and urgency of urine
Hydrangea Renal colic
Parelra Brava Useful in renal colic, prostatic affection and catarrh of the bladder
Rubia Tinct Cures bladder stones
Solidago Renal coilic with dysuria
Sarsaparilla Renal colic
Urtica Urens Helps control urinary tact infection
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
K FLUSH
belladonna, berberls vulgarls, cantharis, , hydrangea, parelra brava, rubla tinct, solidago, sarsaparilla, urtica urens syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82797-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BELLADONNA LEAF (UNII: 6GZW20TIOI) (BELLADONNA LEAF - UNII:6GZW20TIOI) BELLADONNA LEAF 0.12 mg in 120 mL HYDRANGEA ARBORESCENS BARK (UNII: MRX3W33KCB) (HYDRANGEA ARBORESCENS BARK - UNII:MRX3W33KCB) HYDRANGEA ARBORESCENS BARK 0.492 mg in 120 mL URTICA URENS FLOWER (UNII: 3641X02D5V) (URTICA URENS FLOWER - UNII:3641X02D5V) URTICA URENS FLOWER 0.24 mg in 120 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 0.24 mg in 120 mL SOLIDAGO CANADENSIS FLOWERING TOP (UNII: ZHL562L3PR) (SOLIDAGO CANADENSIS FLOWERING TOP - UNII:ZHL562L3PR) SOLIDAGO CANADENSIS FLOWERING TOP 0.24 mg in 120 mL BERBERIS VULGARIS FRUIT (UNII: 6XEF22AHC3) (BERBERIS VULGARIS FRUIT - UNII:6XEF22AHC3) BERBERIS VULGARIS FRUIT 1.7 mg in 120 mL LYTTA VESICATORIA (UNII: 3Q034RO3BT) (LYTTA VESICATORIA - UNII:3Q034RO3BT) LYTTA VESICATORIA 0.492 mg in 120 mL RUBIA CORDIFOLIA ROOT (UNII: 4V873H15CG) (RUBIA CORDIFOLIA ROOT - UNII:4V873H15CG) RUBIA CORDIFOLIA ROOT 0.24 mg in 120 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.06 mg in 120 mL SUCROSE (UNII: C151H8M554) 42 mg in 120 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.42 mg in 120 mL SORBITOL SOLUTION 70% (UNII: 8KW3E207O2) 18 mL in 120 mL GLYCERIN (UNII: PDC6A3C0OX) 12 mL in 120 mL WATER (UNII: 059QF0KO0R) 120 mL in 120 mL .ALPHA.-CELLULOSE (UNII: I355QGZ19A) 0.48 mg in 120 mL Product Characteristics Color pink Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82797-125-12 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/04/2024 Labeler - Dr. Masood Homeopathic Pharmaceuticals Private Limited (645453119) Registrant - Dr, Masood Homeopathic Pharmaceuticals Private Limited (645453119) Establishment Name Address ID/FEI Business Operations Dr. Masood Homeopathic Pharmaceuticals Private Limited 645453119 manufacture(82797-125) , pack(82797-125) , label(82797-125)