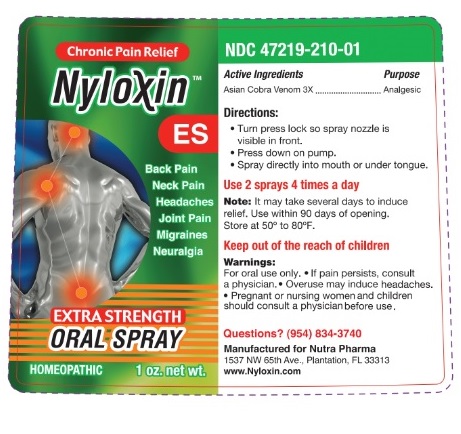
Label: NYLOXIN- naja naja venom spray, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 47219-210-01 - Packager: Nutra Pharma Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses:
-
Warnings:
- Side effects my include headache, nausea, sore throat, allergic rhinitis, gastrointestinal discomfort, itchiness or mild rash.
- If symptoms persist or worsen, stop using this product and consult a physician.
- When Using This Product: avoid contact with the eyes. If product gets into eyes, flush with water. Seek medical attention.
- KEEP OUT OF REACH OF CHILDREN
- Directions for Use:
-
Other Information:
- Do not use if tamper-proof tab is broken.
- This product is not intended to treat disease, it can provide a temporary level of comfort, relief and a feeling of wellness.
- This product has been determined to be safe and effective for moderate to severe (Stage 3) chronic pain, as indicated by the Homeopathic Pharmacopeia of the United States.
- Clinical experience suggests Nyloxin may provide relief from other forms of pain.
- ASK DOCTOR
- Inactive Ingredients:
- QUESTIONS
- Product label
-
INGREDIENTS AND APPEARANCE
NYLOXIN
naja naja venom spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47219-210 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM PHOSPHATE (UNII: SE337SVY37) XYLITOL (UNII: VCQ006KQ1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47219-210-01 1 in 1 BOX 08/23/2010 1 30 mL in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/23/2010 Labeler - Nutra Pharma Corporation (141236286)