Label: OCLUVET NAC NUTRITIONAL LUBRICANT EYE DROPS solution/ drops
- NDC Code(s): 86202-0090-1
- Packager: AMBIENCE FAMILY INC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNINGS AND PRECAUTIONS
Not for use in humans. Keep out of reach of children. Protective gloves should be used and care should be taken when handling the product to avoid skin and eye exposure and accidental ingestion. Accidental exposure may result in skin and eye irritation. Accidental ingestion may cause gastrointestinal disturbances and hypersensitivity reactions in humans.
Warnings: Cease use and consult your veterinarian if the patient experiences eye redness or discomfort for more than 72 hours.Do not use if seal is broken. Do not touch the bottle tip to any surface during application. Do not share this container or the contents with others. Keep out of reach of children.
- DOSAGE & ADMINISTRATION
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
-
WARNINGS AND PRECAUTIONS
Not for use in humans. Keep out of reach of children. Protective gloves should be used and care should be taken when handling the product to avoid skin and eye exposure and accidental ingestion. Accidental exposuremay result in skin and eye irritation. Accidental ingestion may cause gastrointestinal disturbances and hypersensitivity reactions in humans.
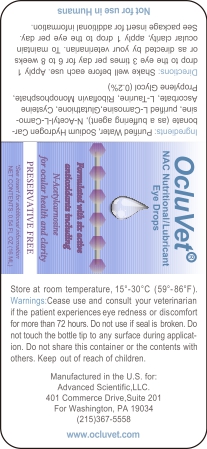
-
PRINCIPAL DISPLAY PANEL
Directions: Shake well before each use.Apply drop to the eye 3 times per day for 6 to 8 weeks or as directed by your veterinarian. To maintain ocular clarity, aooly 1 drop to the eye per day. See package insert for additional information. Ingredients: Purified Water,Sodium Hydrogen Carbonate (as a buffering agent), N-Acetyl-L-Carnosine, purifiedL Carnosine, Glutathione, Cysteine Ascorbate, L-Taurine, Riboflavin Monophosphate, PropyleneGlycol(0.2%) Warnings: Cease use and consult your veterinarian if the patient experiences eye redness or discomfort for more than 72 hours.Do not use if seal is broken. Do not touch the bottle tip to any surface during application. Do not share this container or the contents with others. Keep out of reach of children.
The labeling shows as below:

-
INGREDIENTS AND APPEARANCE
OCLUVET NAC NUTRITIONAL LUBRICANT EYE DROPS
ocluvet nac nutritional lubricant eye drops solution/ dropsProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86202-0090 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength N-ACETYLCARNOSINE (UNII: 0TPN86OQIF) (N-ACETYLCARNOSINE - UNII:0TPN86OQIF) N-ACETYLCARNOSINE 1 mg in 16 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARNOSINE (UNII: 8HO6PVN24W) GLUTATHIONE (UNII: GAN16C9B8O) CYSTEINE (UNII: K848JZ4886) CALCIUM ASCORBATE (UNII: 183E4W213W) TAURINE (UNII: 1EQV5MLY3D) RIBOFLAVIN (UNII: TLM2976OFR) RIBOFLAVIN SODIUM (UNII: 04SRR95RP8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86202-0090-1 16 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/01/2024 Labeler - AMBIENCE FAMILY INC (084561491) Establishment Name Address ID/FEI Business Operations AMBIENCE FAMILY INC 084561491 manufacture, api manufacture

