Label: ACETAMINOPHEN, DEXTROMETHORPHAN HBR, CHLORPHENIRAMINE MALEATE kit
- NDC Code(s): 51316-604-05, 51316-605-05, 51316-609-07
- Packager: CVS Pharmacy, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
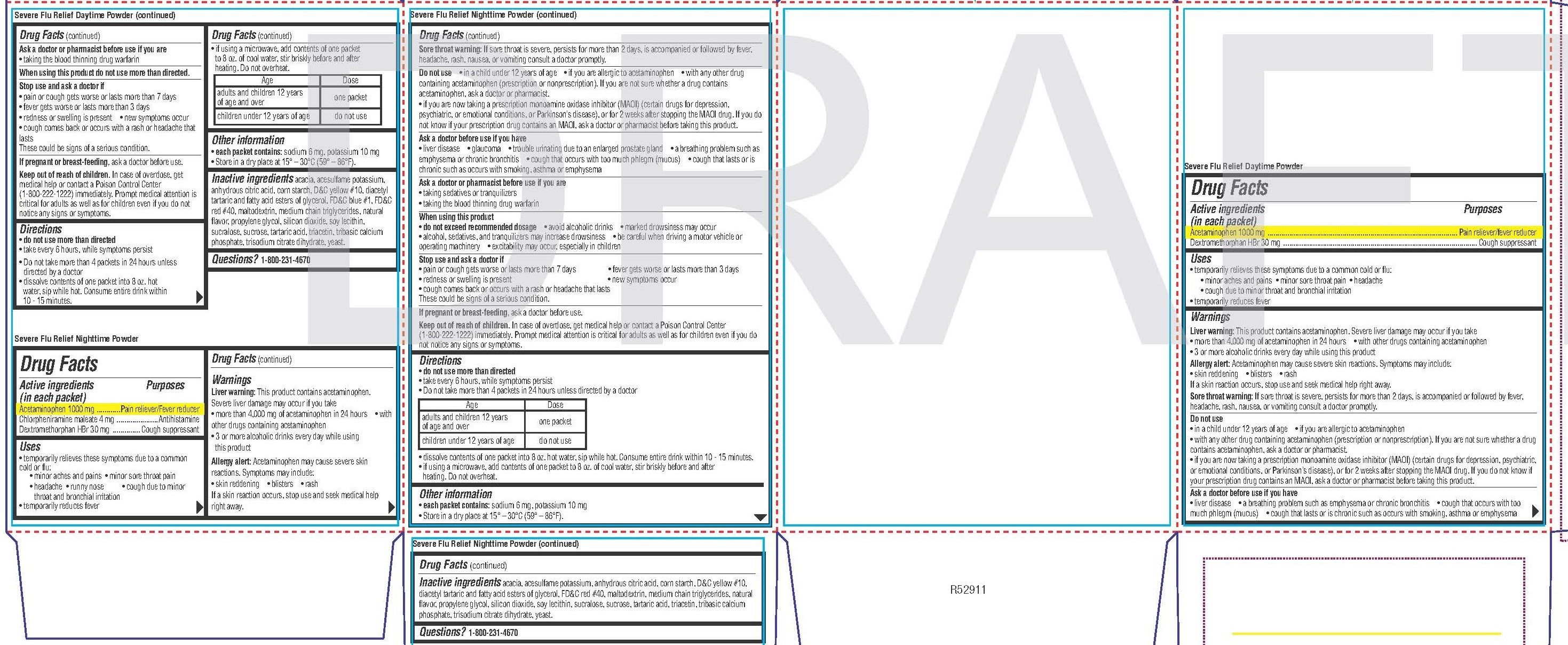
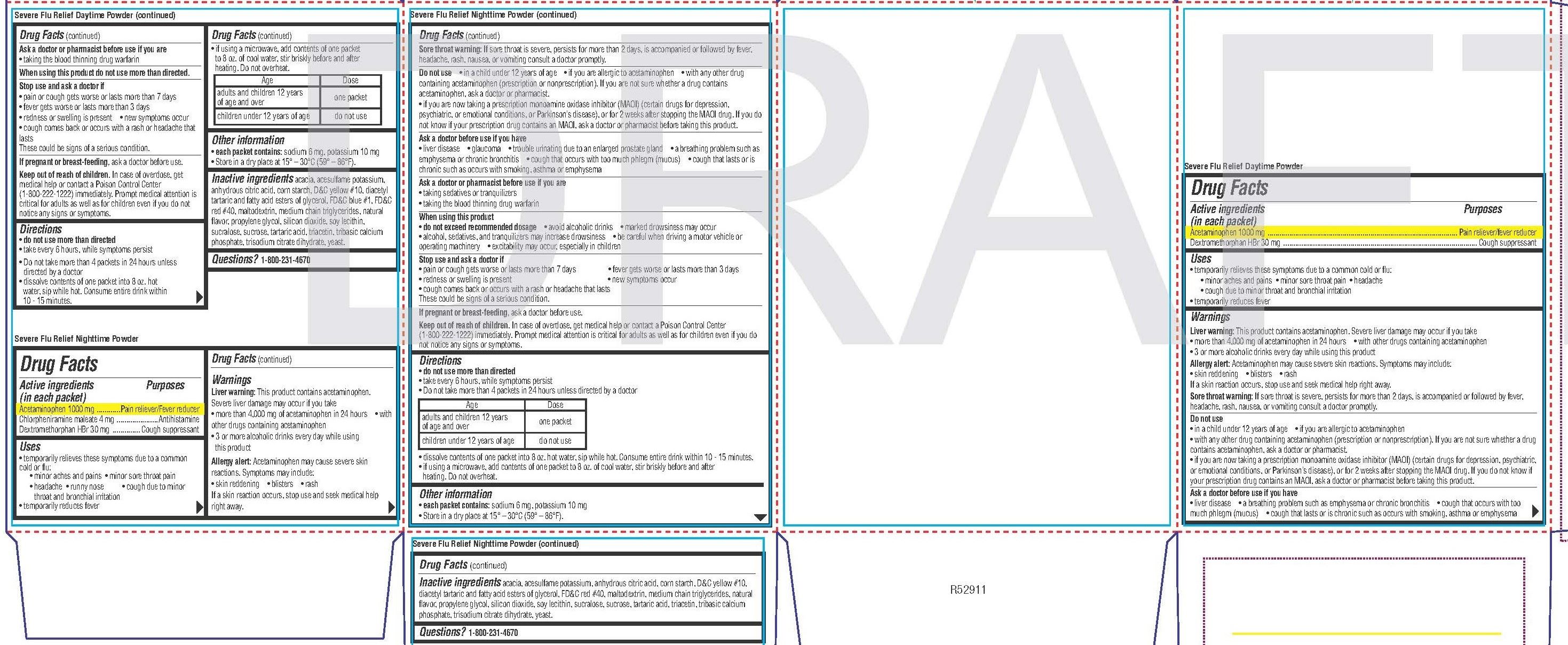
Day Time Powder
Uses
• temporarily relieves these symptoms due to a common cold or flu:
• minor aches and pains • minor sore throat pain • headache
• cough due to minor throat and bronchial irritation
• temporarily reduces feverLiver warning: This product contains acetaminophen. Severe liver damage may occur if you take
• more than 4,000 mg of acetaminophen in 24 hours• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
• skin reddening • blisters • rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly.• in a child under 12 years of age
• if you are allergic to acetaminophen
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.Ask a doctor before use if you have
• liver disease• a breathing problem such as emphysema or chronic bronchitis
• cough that occurs with too much phlegm (mucus)
• cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Directions
• do not use more than directed
• take every 6 hours, while symptoms persist
• Do not take more than 4 packets in 24 hours unless directed by a doctor
• dissolve contents of one packet into 8 oz. hot water, sip while hot. Consume entire drink within 10 - 15 minutes.• if using a microwave, add contents of one packet to 8 oz. of cool water, stir briskly before and after heating. Do not overheat.
Age Dose adults and children 12 years
of age and overone packet children under 12 years of age do not use Other information
• each packet contains: sodium 6 mg, potassium 10 mg
• Store in a dry place at 15° – 30°C (59° – 86°F).acacia, acesulfame potassium, anhydrous citric acid, corn starch, D&C yellow #10, diacetyl tartaric and fatty acid esters of glycerol, FD&C blue #1, FD&C red #40, maltodextrin, medium chain triglycerides, natural flavor, propylene glycol, silicon dioxide, soy lecithin,
sucralose, sucrose, tartaric acid, triacetin, tribasic calcium phosphate, trisodium citrate dihydrate, yeast. -
Nighttime Powder
Drug Facts
Active ingredients (in each packet)
Acetaminophen 1000 mg
Chlorpheniramine maleate 4 mg
Dextromethorphan HBr 30 mgUses
• temporarily relieves these symptoms due to a common cold or flu:
• minor aches and pains • minor sore throat pain
• headache • runny nose • cough due to minor throat and bronchial irritation
• temporarily reduces feverLiver warning: This product contains acetaminophen. Severe liver damage may occur if you take
• more than 4,000 mg of acetaminophen in 24 hours• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
• skin reddening • blisters • rash
If a skin reaction occurs, stop use and seek medical help right away.Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly
Do not use
• in a child under 12 years of age
• if you are allergic to acetaminophen
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.Ask a doctor before use if you have
• liver disease• glaucoma
• trouble urinating due to an enlarged prostate gland
• a breathing problem such as emphysema or chronic bronchitis
• cough that occurs with too much phlegm (mucus) • cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Ask a doctor or pharmacist before use if you are
• taking sedatives or tranquilizers
• taking the blood thinning drug warfarinWhen using this product
• do not exceed recommended dosage• avoid alcoholic drinks
• marked drowsiness may occur
• alcohol, sedatives, and tranquilizers may increase drowsiness• be careful when driving a motor vehicle or operating machinery
• excitability may occur, especially in children
Directions
• do not use more than directed
• take every 6 hours, while symptoms persist
• Do not take more than 4 packets in 24 hours unless directed by a doctor• dissolve contents of one packet into 8 oz. hot water, sip while hot. Consume entire drink within 10 - 15 minutes.
• if using a microwave, add contents of one packet to 8 oz. of cool water, stir briskly before and after heating. Do not overheat.Age Dose adults and children 12 years
of age and overone packet children under 12 years of age do not use Other information
• each packet contains: sodium 6 mg, potassium 10 mg
• Store in a dry place at 15° – 30°C (59° – 86°F).acacia, acesulfame potassium, anhydrous citric acid, corn starch, D&C yellow #10, diacetyl tartaric and fatty acid esters of glycerol, FD&C red #40, maltodextrin, medium chain triglycerides, natural flavor, propylene glycol, silicon dioxide, soy lecithin, sucralose, sucrose, tartaric acid, triacetin, tribasic calcium phosphate, trisodium citrate dihydrate, yeast.
- QUESTIONS
- Draft Label
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN, DEXTROMETHORPHAN HBR, CHLORPHENIRAMINE MALEATE
acetaminophen, dextromethorphan hbr, chlorpheniramine maleate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-609 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-609-07 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 04/05/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 6 in 12 Part 2 1 PACKET 6 in 12 Part 1 of 2 ACETAMINOPHEN, CHLORPHENIRAMINE MALEATE, DEXTROMETHORPHAN HBR
acetaminophen, chlorpheniramine maleate, dextromethorphan hbr powderProduct Information Item Code (Source) NDC:51316-605 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 1000 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DIACETYLTARTARIC AND FATTY ACID ESTERS OF GLYCEROL (UNII: 248HN3Z28U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) YEAST (UNII: 3NY3SM6B8U) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) ACACIA (UNII: 5C5403N26O) FD&C RED NO. 40 (UNII: WZB9127XOA) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SUCRALOSE (UNII: 96K6UQ3ZD4) TRIACETIN (UNII: XHX3C3X673) SOYBEAN LECITHIN (UNII: 1DI56QDM62) Product Characteristics Color yellow (Light Yellow) Score Shape Size Flavor HONEY (Honey Lemon) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-605-05 6 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/05/2024 Part 2 of 2 ACETAMINOPHEN, DEXTROMETHORPHAN HBR
acetaminophen, dextromethorphan hbr powderProduct Information Item Code (Source) NDC:51316-604 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 1000 mg Inactive Ingredients Ingredient Name Strength TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) STARCH, CORN (UNII: O8232NY3SJ) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MALTODEXTRIN (UNII: 7CVR7L4A2D) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIACETYLTARTARIC AND FATTY ACID ESTERS OF GLYCEROL (UNII: 248HN3Z28U) YEAST (UNII: 3NY3SM6B8U) SUCROSE (UNII: C151H8M554) TRIACETIN (UNII: XHX3C3X673) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCRALOSE (UNII: 96K6UQ3ZD4) ACACIA (UNII: 5C5403N26O) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) SOYBEAN LECITHIN (UNII: 1DI56QDM62) FD&C RED NO. 40 (UNII: WZB9127XOA) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TARTARIC ACID (UNII: W4888I119H) Product Characteristics Color yellow (Light yellow) Score Shape Size Flavor HONEY (Honey Lemon) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-604-05 6 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/05/2024 Labeler - CVS Pharmacy, Inc (062312574)