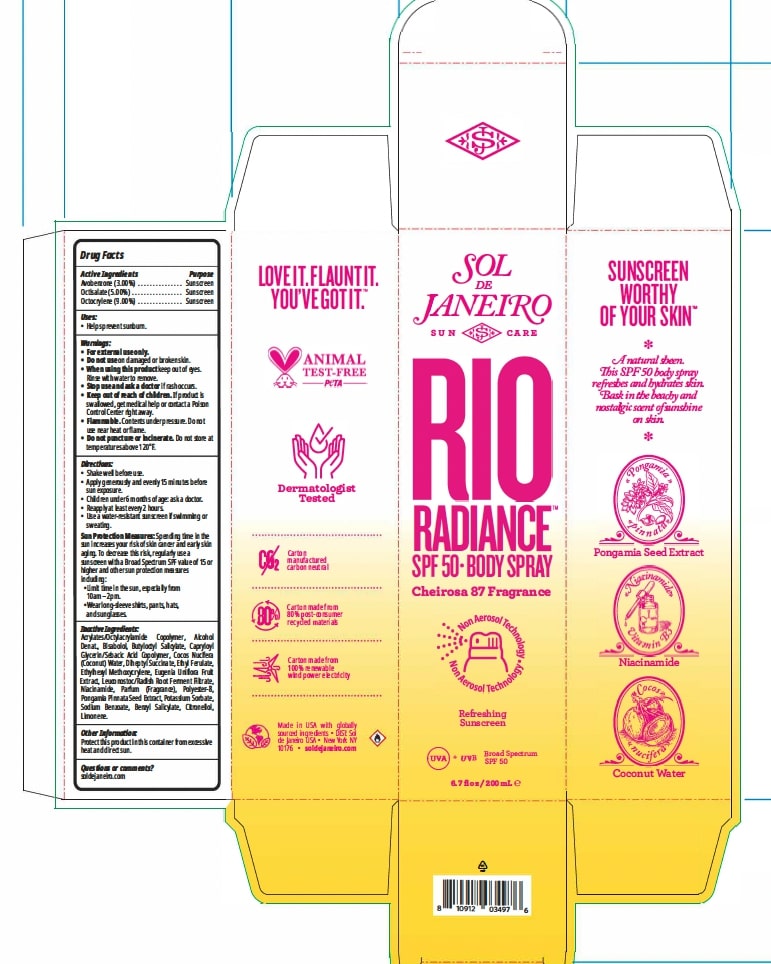
Label: RIO RADIANCE SPF 50 BODYSPRAY- octocrylene, octisalate, avobenzone aerosol, spray
- NDC Code(s): 83982-1001-1
- Packager: Sol de Janeiro USA, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warnings:
• For external use only.
• Do not use on damaged or broken skin.
• When using this product keep out of eyes. Rinse with water to
remove.
• Stop use and ask a doctor if rash occurs.
• Keep out of reach of children. If product is swallowed, get
medical help or contact a Poison Control Center right away.• Flammable. Contents under pressure. Do not use near heat or flame.
• Do not puncture or incinerate. Do not store at temperatures above 120°F.
Sun Protection Measures: Spending time in the sun increases
your risk of skin cancer and early skin aging. To decrease this risk,
regularly use a sunscreen with a Broad Spectrum SPF value of 15 or
higher and other sun protection measures including:
• Limit time in the sun, especially from 10am - 2pm.
• Wear long-sleeved shirts, pants, hats, and sunglasses.
-
INACTIVE INGREDIENT
Acrylates/Octylacrylamide Copolymer, Alcohol Denat., Bisabolol, Butyloctyl Salicylate, Capryloyl Glycerin/Sebacic Acid Copolymer, Cocos Nucifera (Coconut) Water, Diheptyl Succinate, Ethyl Ferulate, Ethylhexyl Methoxycrylene, Eugenia Uniflora Fruit Extract, Leuconostoc/Radish Root Ferment Filtrate, Niacinamide, Parfum (Fragrance), Polyester-8, Pongamia Pinnata Seed Extract, Potassium Sorbate, Sodium Benzoate, Benzyl Salicylate, Citronellol, Limonene.
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RIO RADIANCE SPF 50 BODYSPRAY
octocrylene, octisalate, avobenzone aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83982-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength EUGENIA UNIFLORA FRUIT (UNII: 39OSC52QCD) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) SODIUM BENZOATE (UNII: OJ245FE5EU) ALCOHOL (UNII: 3K9958V90M) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) DIHEPTYL SUCCINATE (UNII: 057N7SS26Y) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ACRYLATES/OCTYLACRYLAMIDE COPOLYMER (40000 MW) (UNII: 7LL6SY9YFV) ETHYL FERULATE (UNII: 5B8915UELW) NIACINAMIDE (UNII: 25X51I8RD4) LEVOMENOL (UNII: 24WE03BX2T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) COCONUT WATER (UNII: 267F5Y81NT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83982-1001-1 1 in 1 CARTON 03/27/2024 1 200 mL in 1 CANISTER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/27/2024 Labeler - Sol de Janeiro USA, Inc (080098027)