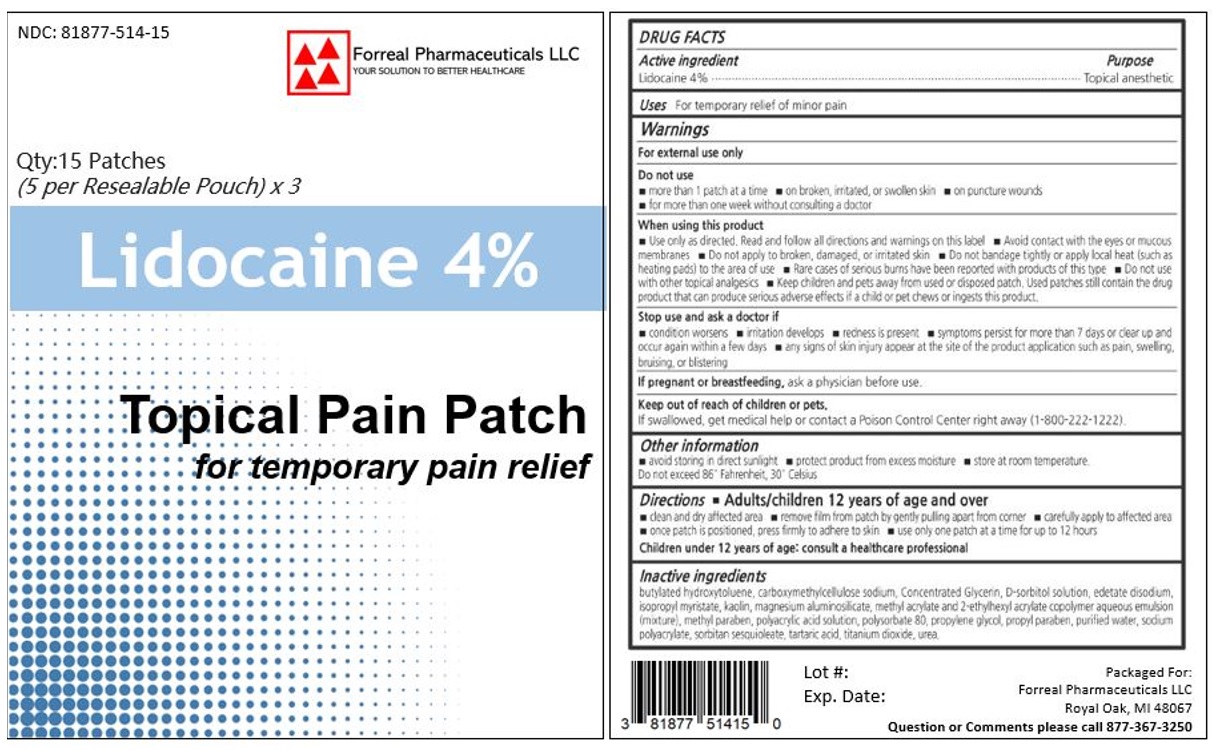
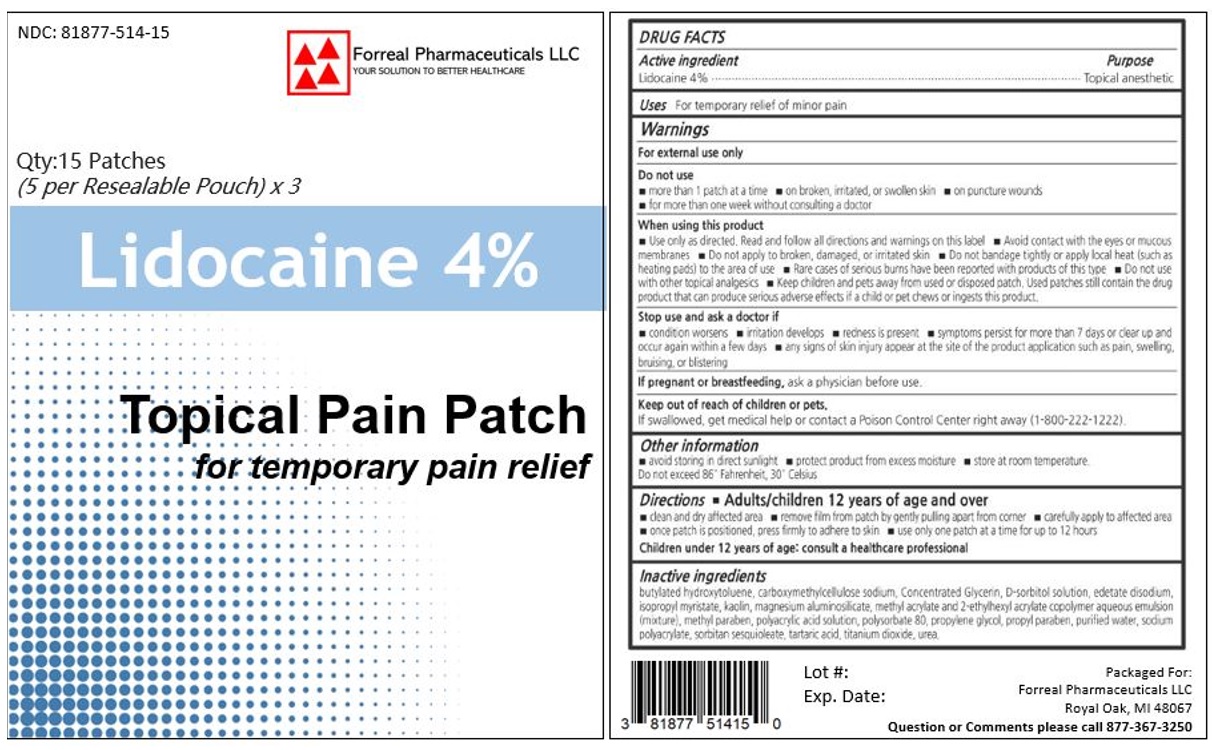
Label: LIDOCAINE patch
- NDC Code(s): 81877-514-15
- Packager: FORREAL PHARMACEUTICALS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Adults and children 12 years of age and over:
- Clean and dry the affected area
- Open pouch and remove one patch
- Apply 1 patch at a time to affected area; not more than 3 to 4 times daily
- Reseal pouch containing unused patches after each use
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: Consult a doctor
- Other information:
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81877-514 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) KAOLIN (UNII: 24H4NWX5CO) SILODRATE ANHYDROUS (UNII: 59110L5W24) 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) METHYLPARABEN (UNII: A2I8C7HI9T) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) UREA (UNII: 8W8T17847W) Product Characteristics Color Score Shape RECTANGLE (White Flexible Patch) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81877-514-15 3 in 1 BOX 06/27/2022 1 1 g in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/27/2022 Labeler - FORREAL PHARMACEUTICALS LLC (118029197)