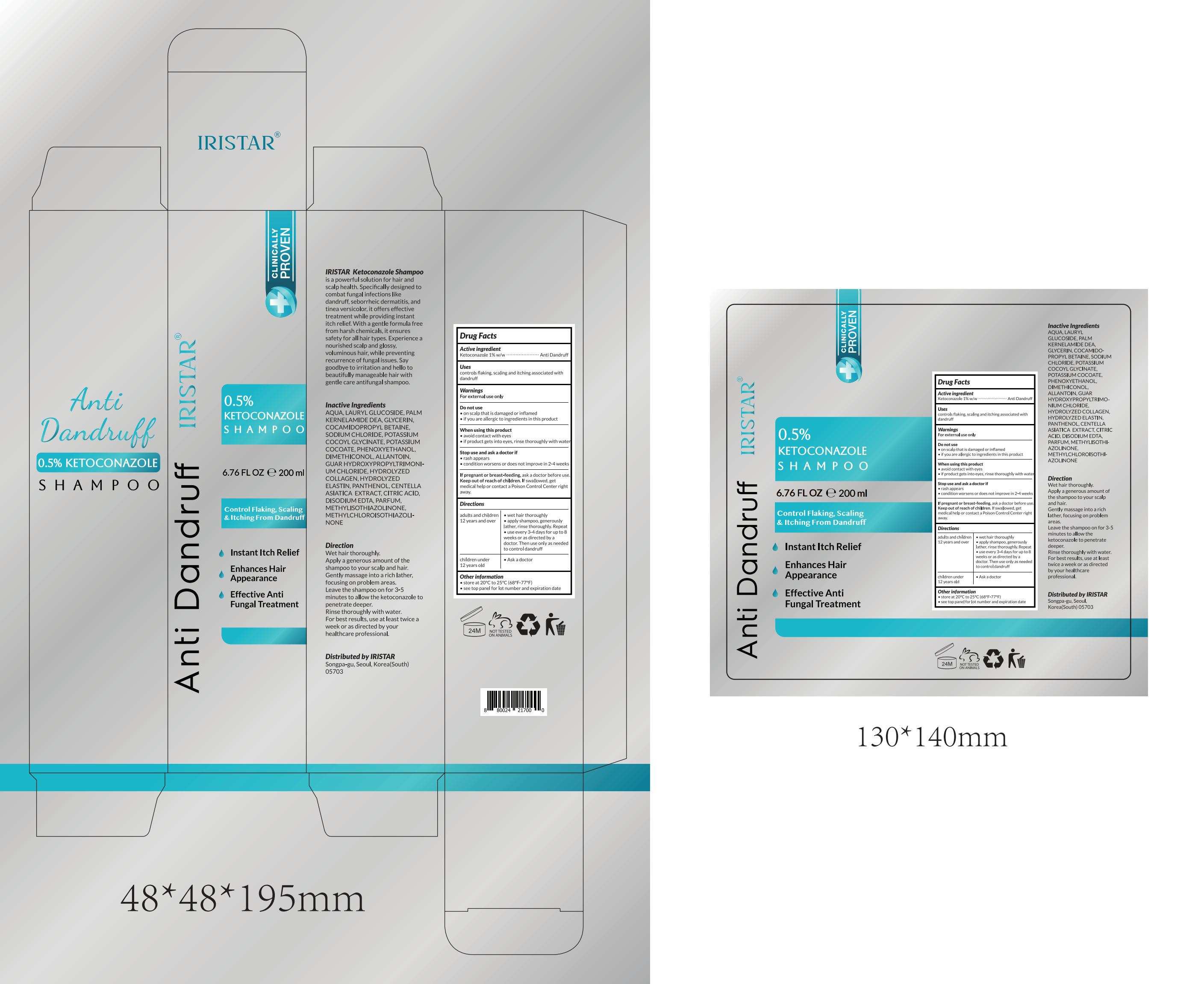
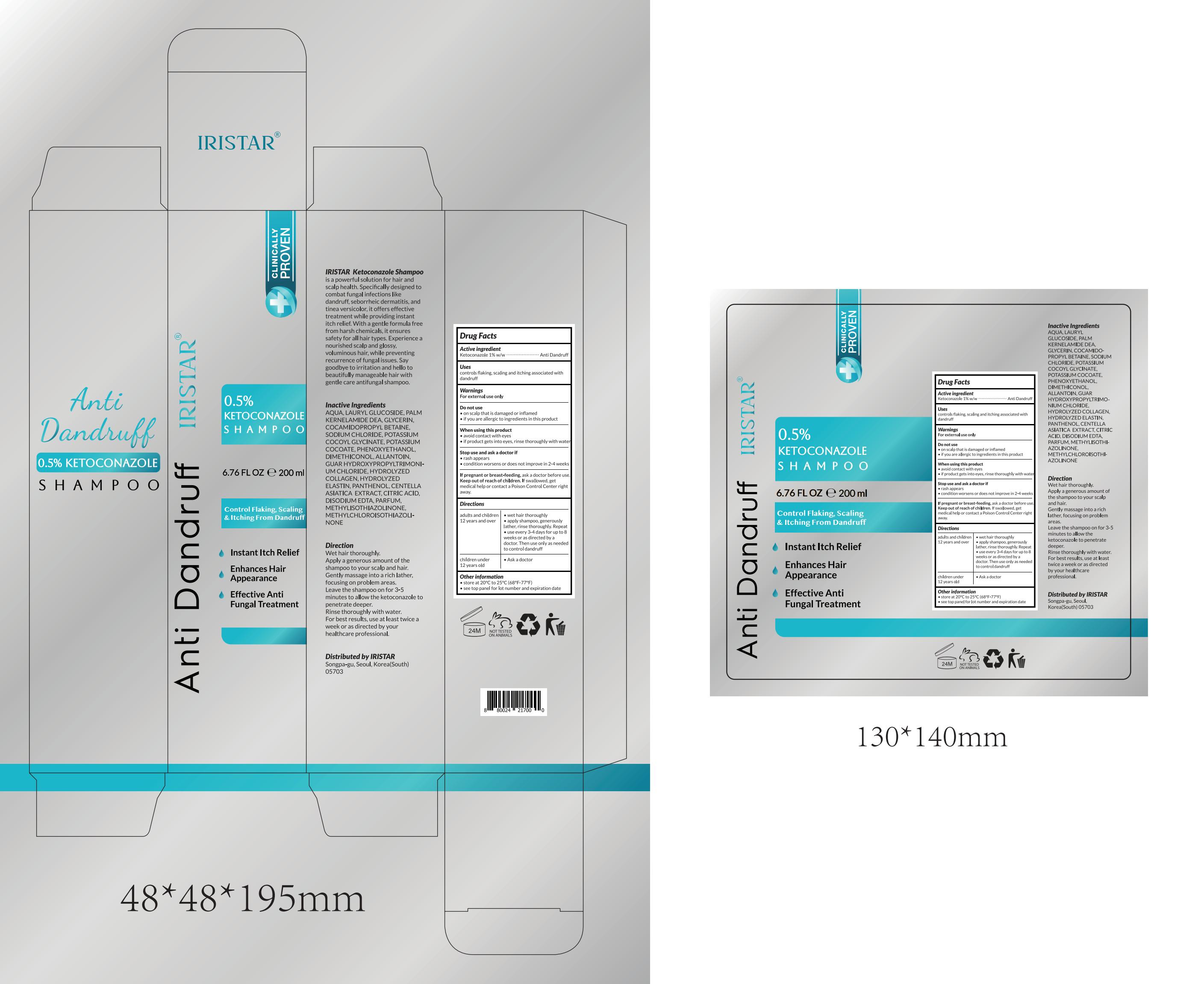
Label: IRISTAR ANTI DANDRUFF SHAMPOO.- ketoconazole shampoo lotion/shampoo
- NDC Code(s): 83462-018-01
- Packager: Eubizrival LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
-
Inactive ingredients
AQUA, LAURYL GLUCOSIDE, PALM KERNELAMIDE DEA, GLYCERIN, COCAMIDOPROPYL BETAINE, SODIUM CHLORIDE, POTASSIUM COCOYL GLYCINATE, POTASSIUM COCOATE, PHENOXYETHANOL, DIMETHICONOL, ALLANTOIN, GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE, HYDROLYZED COLLAGEN, HYDROLYZED ELASTIN, PANTHENOL, CENTELLA ASIATICA EXTRACT, CITRIC ACID, DISODIUM EDTA, PARFUM, METHYLISOTHIAZOLINONE, METHYLCHLOROISOTHIAZOLINONE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IRISTAR ANTI DANDRUFF SHAMPOO.
ketoconazole shampoo lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83462-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOCONAZOLE (UNII: R9400W927I) (KETOCONAZOLE - UNII:R9400W927I) KETOCONAZOLE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) ALLANTOIN (UNII: 344S277G0Z) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POTASSIUM COCOYL GLYCINATE (UNII: WZ70FUF22U) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) PANTHENOL (UNII: WV9CM0O67Z) PALM KERNEL OIL (UNII: B0S90M0233) SODIUM CHLORIDE (UNII: 451W47IQ8X) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) DIMETHICONOL (14000 CST) (UNII: M2HW98ZA4V) HYDROLYSED BOVINE COLLAGEN (ENZYMATIC; 2000-5000 MW) (UNII: 5WE8P977RQ) HYDROLYZED BOVINE ELASTIN (BASE; 1000 MW) (UNII: ZR28QKN0WT) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83462-018-01 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 03/29/2024 Labeler - Eubizrival LLC (036572203) Establishment Name Address ID/FEI Business Operations Eubizrival LLC 036572203 label(83462-018) , manufacture(83462-018)