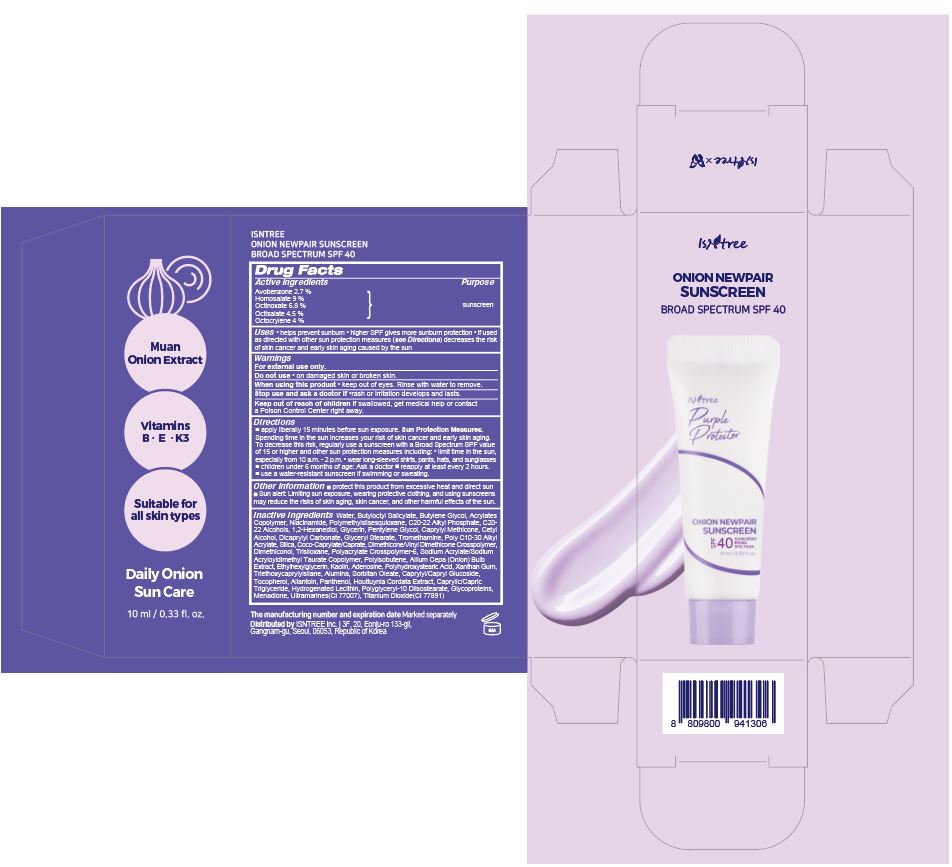
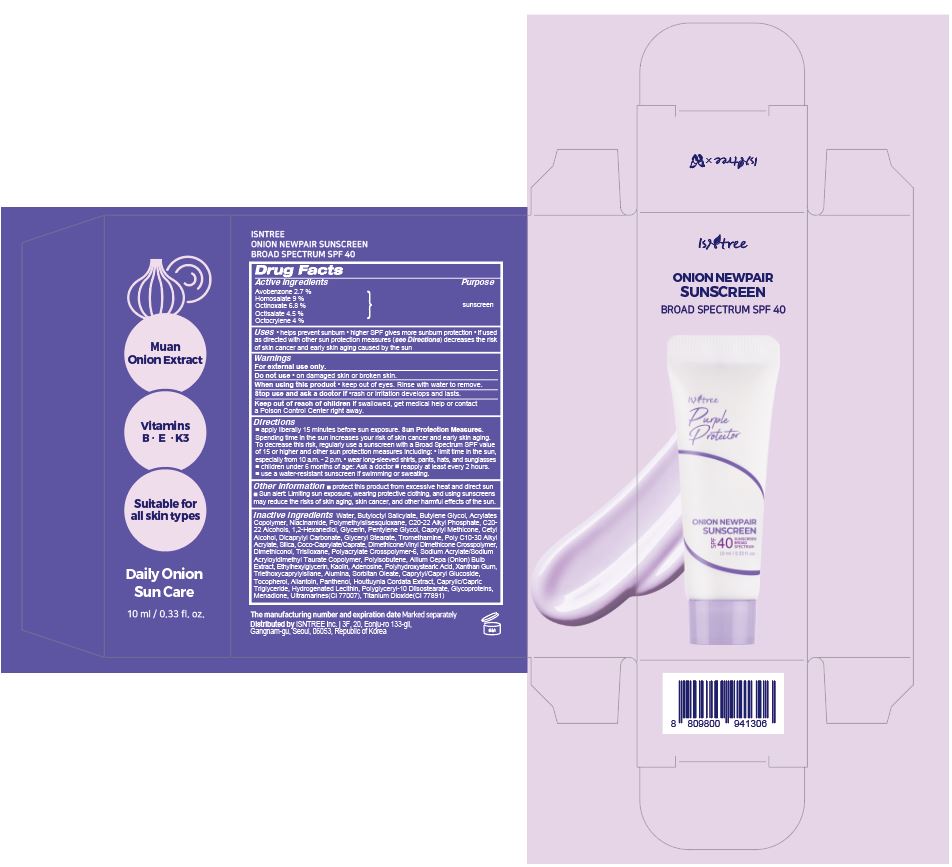
Label: ISNTREE ONION NEWPAIR SUNSCREEN- avobenzone, homosalate, octinoxate, octisalate, octocrylene cream
- NDC Code(s): 81100-102-01, 81100-102-02
- Packager: ISNTREE Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
■ apply liberally 15 minutes before sun exposure. Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection measures including: • limit time
in the sun, especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants,
hats, and sunglasses ■ children under 6 months of age: Ask a doctor ■ reapply
at least every 2 hours. ■ use a water-resistant sunscreen if swimming or
sweating.
- Other information
-
Inactive ingredients
Water, Butyloctyl Salicylate, Butylene Glycol, Acrylates Copolymer, Niacinamide, Polymethylsilsesquioxane, C20-22 Alkyl Phosphate, C20-22 Alcohols, 1,2-Hexanediol, Glycerin, Pentylene Glycol, Caprylyl Methicone, Cetyl Alcohol, Dicaprylyl Carbonate, Glyceryl Stearate, Tromethamine, Poly C10-30 Alkyl Acrylate, Silica, Coco-Caprylate/Caprate, Dimethicone/Vinyl Dimethicone Crosspolymer, Dimethiconol, Trisiloxane, Polyacrylate Crosspolymer-6, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polyisobutene, Allium Cepa (Onion) Bulb Extract, Ethylhexylglycerin, Kaolin, Adenosine, Polyhydroxystearic Acid, Xanthan Gum, Triethoxycaprylylsilane, Alumina, Sorbitan Oleate, Caprylyl/Capryl Glucoside, Tocopherol, Allantoin, Panthenol, Houttuynia Cordata Extract, Caprylic/Capric Triglyceride, Hydrogenated Lecithin, Polyglyceryl-10 Diisostearate, Glycoproteins, Menadione, Ultramarines(CI 77007), Titanium Dioxide(CI 77891)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ISNTREE ONION NEWPAIR SUNSCREEN
avobenzone, homosalate, octinoxate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81100-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.9 g in 10 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.68 g in 10 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.45 g in 10 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.4 g in 10 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.27 g in 10 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) C20-22 ALCOHOLS (UNII: O4M0347C6A) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) ALLANTOIN (UNII: 344S277G0Z) PANTHENOL (UNII: WV9CM0O67Z) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) C20-22 ALKYL PHOSPHATE (UNII: L4VKP0Y7RP) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) TOCOPHEROL (UNII: R0ZB2556P8) TROMETHAMINE (UNII: 023C2WHX2V) PENTYLENE GLYCOL (UNII: 50C1307PZG) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TRISILOXANE (UNII: 9G1ZW13R0G) ONION (UNII: 492225Q21H) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) ADENOSINE (UNII: K72T3FS567) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) KAOLIN (UNII: 24H4NWX5CO) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) MENADIONE (UNII: 723JX6CXY5) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81100-102-02 1 in 1 BOX 03/27/2024 1 NDC:81100-102-01 10 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/27/2024 Labeler - ISNTREE Inc. (689043012) Establishment Name Address ID/FEI Business Operations Kolmar Korea Co.,LTD. Gwanjeong 2 factory 963271750 manufacture(81100-102)