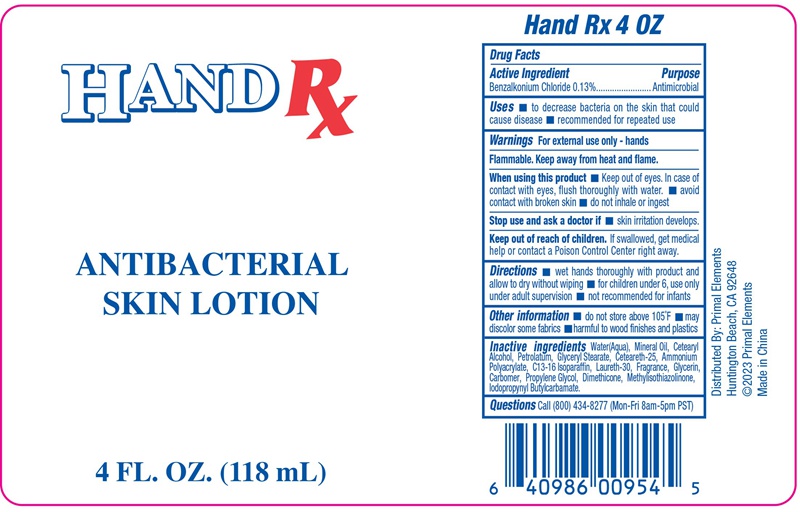
Label: ANTIBACTERIAL SKIN- benzalkonium chloride lotion
- NDC Code(s): 76176-207-01
- Packager: NINGBO LIYUAN DAILY CHEMICAL PRODUCTS CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL SKIN
benzalkonium chloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76176-207 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) C13-16 ISOPARAFFIN (UNII: LED42LZG6O) LAURETH-30 (UNII: W9D845551A) CARBOMER 940 (UNII: 4Q93RCW27E) AMMONIUM POLYACRYLOYLDIMETHYL TAURATE (UNII: F01RIY4371) MINERAL OIL (UNII: T5L8T28FGP) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) PETROLATUM (UNII: 4T6H12BN9U) CETEARETH-25 (UNII: 8FA93U5T67) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76176-207-01 118 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 03/20/2024 Labeler - NINGBO LIYUAN DAILY CHEMICAL PRODUCTS CO., LTD. (530766098)