Label: SOTALOL HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-4435-0, 54868-4435-1, 54868-4435-2, 54868-4435-3, view more54868-5549-0, 54868-5549-1, 54868-5614-0, 54868-5614-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0185-0170, 0185-0171, 0185-0177
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 23, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
To minimize the risk of induced arrhythmia, patients initiated or re-initiated on sotalol should be placed for a minimum of three days (on their maintenance dose) in a facility that can provide cardiac resuscitation and continuous electrocardiographic monitoring. Creatinine clearance should be calculated prior to dosing. For detailed instructions regarding dose selection and special cautions for people with renal impairment, see DOSAGE AND ADMINISTRATION. Sotalol is also indicated for the maintenance of normal sinus rhythm [delay in time to recurrence of atrial fibrillation/atrial flutter (AFIB/AFL)] in patients with symptomatic AFIB/AFL who are currently in sinus rhythm and is marketed under the brand name BETAPACE AF™. Sotalol hydrochloride tablets are not approved for the AFIB/AFL indication and should not be substituted for BETAPACE AF™ because only BETAPACE AF™ is distributed with a patient package insert that is appropriate for patients with AFIB/AFL.
-
DESCRIPTION
Sotalol hydrochloride USP is an antiarrhythmic drug with Class II (beta-adrenoreceptor blocking) and Class III (cardiac action potential duration prolongation) properties. It is supplied as a light-blue, capsule-shaped tablet for oral administration. Sotalol hydrochloride USP is a white, crystalline solid with a molecular weight of 308.8. It is hydrophilic, soluble in water, propylene glycol and ethanol, but is only slightly soluble in chloroform. Chemically, sotalol hydrochloride USP is d,l-N-[4-[1-hydroxy-2-[(1-methylethyl) amino]ethyl]phenyl]methane-sulfonamide monohydrochloride. The molecular formula is C12H20N2O3S•HCl and is represented by the following structural formula:

Each sotalol hydrochloride tablet USP, for oral administration, contains 80 mg, 120 mg, 160 mg or 240 mg of sotalol hydrochloride. Each tablet also contains the following inactive ingredients: colloidal silicon dioxide, FD&C blue No. 1 aluminum lake, hydroxypropyl cellulose, lactose anhydrous, lactose monohydrate, magnesium stearate, pregelatinized starch and sodium starch glycolate.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Sotalol hydrochloride tablets have both beta-adrenoreceptor blocking (Vaughan Williams Class II) and cardiac action potential duration prolongation (Vaughan Williams Class III) antiarrhythmic properties. Sotalol hydrochloride tablets are a racemic mixture of d- and l-sotalol. Both isomers have similar Class III antiarrhythmic effects, while the I-isomer is responsible for virtually all of the beta-blocking activity. The beta-blocking effect of sotalol is non-cardioselective, half maximal at about 80 mg/day and maximal at doses between 320 mg/day and 640 mg/day. Sotalol does not have partial agonist or membrane stabilizing activity. Although significant beta-blockade occurs at oral doses as low as 25 mg, significant Class III effects are seen only at daily doses of 160 mg and above.
In children, a Class III electrophysiologic effect can be seen at daily doses of 210 mg/m2 body surface area (BSA). A reduction of the resting heart rate due to the beta-blocking effect of sotalol is observed at daily doses ≥ 90 mg/m2 in children.
Electrophysiology
Sotalol hydrochloride prolongs the plateau phase of the cardiac action potential in the isolated myocyte, as well as in isolated tissue preparations of ventricular or atrial muscle (Class III activity). In intact animals it slows heart rate, decreases AV nodal conduction and increases the refractory periods of atrial and ventricular muscle and conduction tissue.
In man, the Class II (beta-blockade) electrophysiological effects of sotalol hydrochloride tablets are manifested by increased sinus cycle length (slowed heart rate), decreased AV nodal conduction and increased AV nodal refractoriness. The Class III electrophysiological effects in man include prolongation of the atrial and ventricular monophasic action potentials and effective refractory period prolongation of atrial muscle, ventricular muscle and atrio-ventricular accessory pathways (where present) in both the anterograde and retrograde directions. With oral doses of 160 mg/day to 640 mg/day, the surface ECG shows dose-related mean increases of 40 msec to 100 msec in QT and 10 msec to 40 msec in QTc. (See WARNINGSfor description of relationship between QTc and torsade de pointes type arrhythmias.) No significant alteration in QRS interval is observed. In a small study (n=25) of patients with implanted defibrillators treated concurrently with sotalol hydrochloride tablets, the average defibrillatory threshold was 6 joules (range 2 joules to 15 joules) compared to a mean of 16 joules for a non-randomized comparative group primarily receiving amiodarone.
Twenty-five children in an unblinded, multicenter trial with supraventricular (SVT) and/or ventricular (VT) tachyarrhythmias, aged between 3 days and 12 years (mostly neonates and infants), received an ascending titration regimen with daily doses of 30 mg/m2, 90 mg/m2 and 210 mg/m2 with dosing every 8 hours for a total 9 doses. During steady-state, the respective average increases above baseline of the QTc interval, in msec (%), were 2(+1%), 14(+4%) and 29(+7%) msec at the 3 dose levels. The respective mean maximum increases above baseline of the QTc interval, in msec (%), were 23(+6%), 36(+9%) and 55(+14%) msec at the 3 dose levels. The steady-state percent increases in the RR interval were 3%, 9% and 12%. The smallest children (BSA< 0.33m2) showed a tendency for larger Class III effects (ΔQTc) and an increased frequency of prolongations of the QTc interval as compared with larger children (BSA≥0.33m2). The beta-blocking effects also tended to be greater in the smaller children (BSA< 0.33m2). Both the Class III and beta-blocking effects of sotalol were linearly related with the plasma concentrations.
Hemodynamics
In a study of systemic hemodynamic function measured invasively in 12 patients with a mean LV ejection fraction of 37% and ventricular tachycardia (9 sustained and 3 non-sustained), a median dose of 160 mg twice daily of sotalol hydrochloride tablets produced a 28% reduction in heart rate and a 24% decrease in cardiac index at 2 hours post dosing at steady-state. Concurrently, systemic vascular resistance and stroke volume showed non-significant increases of 25% and 8%, respectively. Pulmonary capillary wedge pressure increased significantly from 6.4 mmHg to 11.8 mmHg in the 11 patients who completed the study. One patient was discontinued because of worsening congestive heart failure. Mean arterial pressure, mean pulmonary artery pressure and stroke work index did not significantly change. Exercise and isoproterenol induced tachycardia are antagonized by sotalol hydrochloride tablets, and total peripheral resistance increases by a small amount.
In hypertensive patients, sotalol hydrochloride tablets produces significant reductions in both systolic and diastolic blood pressures. Although sotalol hydrochloride tablets are usually well-tolerated hemodynamically, caution should be exercised in patients with marginal cardiac compensation as deterioration in cardiac performance may occur (see WARNINGS, Congestive Heart Failure).
Clinical Actions
Sotalol hydrochloride tablets have been studied in life-threatening and less severe arrhythmias. In patients with frequent premature ventricular complexes (VPC), sotalol hydrochloride tablets were significantly superior to placebo in reducing VPCs, paired VPCs and non-sustained ventricular tachycardia (NSVT); the response was dose-related through 640 mg/day with 80% to 85% of patients having at least a 75% reduction of VPCs. Sotalol hydrochloride tablets were also superior at the doses evaluated, to propranolol (40 mg to 80 mg TID) and similar to quinidine (200 mg to 400 mg QID) in reducing VPCs. In patients with life-threatening arrhythmias [sustained ventricular tachycardia/fibrillation (VT/VF)], sotalol hydrochloride tablets were studied acutely [by suppression of programmed electrical stimulation (PES) induced VT and by suppression of Holter monitor evidence of sustained VT] and, in acute responders, chronically.
In a double-blind, randomized comparison of sotalol hydrochloride tablets and procainamide given intravenously (total of 2 mg/kg sotalol hydrochloride tablets vs. 19 mg/kg of procainamide over 90 minutes), sotalol hydrochloride tablets suppressed PES induction in 30% of patients vs. 20% for procainamide (p=0.2).
In a randomized clinical trial [Electrophysiologic Study Versus Electrocardiographic Monitoring (ESVEM) Trial] comparing choice of antiarrhythmic therapy by PES suppression vs. Holter monitor selection (in each case followed by treadmill exercise testing) in patients with a history of sustained VT/VF who were also inducible by PES, the effectiveness acutely and chronically of sotalol hydrochloride tablets was compared with 6 other drugs (procainamide, quinidine, mexiletine, propafenone, imipramine and pirmenol). Overall response, limited to first randomized drug, was 39% for sotalol and 30% for the pooled other drugs. Acute response rate for first drug randomized using suppression of PES induction was 36% for sotalol hydrochloride tablets vs. a mean of 13% for the other drugs. Using the Holter monitoring endpoint (complete suppression of sustained VT, 90% suppression of NSVT, 80% suppression of VPC pairs and at least 70% suppression of VPCs), sotalol hydrochloride tablets yielded 41% response vs. 45% for the other drugs combined. Among responders placed on long-term therapy identified acutely as effective (by either PES or Holter), sotalol hydrochloride tablets, when compared to the pool of other drugs, had the lowest two-year mortality (13% vs. 22%), the lowest two-year VT recurrence rate (30% vs. 60%) and the lowest withdrawal rate (38% vs. about 75% to 80%). The most commonly used doses of sotalol hydrochloride tablets in this trial were 320 mg/day to 480 mg/day (66% of patients), with 16% receiving 240 mg/day or less and 18% receiving 640 mg or more.
It cannot be determined, however, in the absence of a controlled comparison of sotalol hydrochloride tablets vs. no pharmacologic treatment (e.g., in patients with implanted defibrillators) whether sotalol hydrochloride tablet response causes improved survival or identifies a population with a good prognosis.
In a large double-blind, placebo controlled secondary prevention (post-infarction) trial (n=1,456), sotalol hydrochloride tablets were given as a non-titrated initial dose of 320 mg once daily. Sotalol hydrochloride tablets did not produce a significant increase in survival (7.3% mortality on sotalol hydrochloride tablets vs. 8.9% on placebo, p=0.3), but overall did not suggest an adverse effect on survival. There was, however, a suggestion of an early (i.e., first 10 days) excess mortality (3% on sotalol vs. 2% on placebo). In a second small trial (n=17 randomized to sotalol) where sotalol was administered at high doses (e.g., 320 mg twice daily) to high-risk post-infarction patients (ejection fraction <40% and either >10 VPC/hr or VT on Holter), there were 4 fatalities and 3 serious hemodynamic/electrical adverse events within two weeks of initiating sotalol.
Pharmacokinetics
In healthy subjects, the oral bioavailability of sotalol hydrochloride tablets is 90% to 100%. After oral administration, peak plasma concentrations are reached in 2.5 to 4 hours and steady-state plasma concentrations are attained within 2 to 3 days (i.e., after 5 to 6 doses when administered twice daily). Over the dosage range 160 mg/day to 640 mg/day sotalol hydrochloride tablets display dose proportionality with respect to plasma concentrations. Distribution occurs to a central (plasma) and to a peripheral compartment, with a mean elimination half-life of 12 hours. Dosing every 12 hours results in trough plasma concentrations which are approximately one-half of those at peak.
Sotalol hydrochloride tablets do not bind to plasma proteins and is not metabolized. Sotalol hydrochloride tablets show very little intersubject variability in plasma levels. The pharmacokinetics of the d and I enantiomers of sotalol are essentially identical. Sotalol hydrochloride tablets cross the blood brain barrier poorly. Excretion is predominantly via the kidney in the unchanged form and therefore lower doses are necessary in conditions of renal impairment (see DOSAGE AND ADMINISTRATION). Age per se does not significantly alter the pharmacokinetics of sotalol hydrochloride tablets, but impaired renal function in geriatric patients can increase the terminal elimination half-life, resulting in increased drug accumulation. The absorption of sotalol hydrochloride tablets was reduced by approximately 20% compared to fasting when it was administered with a standard meal. Since sotalol hydrochloride tablets are not subject to first-pass metabolism, patients with hepatic impairment show no alteration in clearance of sotalol hydrochloride tablets.
The combined analysis of two unblinded, multicenter trials (a single dose and a multiple dose study) with 59 children, aged between 3 days and 12 years, showed the pharmacokinetics of sotalol to be first order. A daily dose of 30 mg/m2 of sotalol was administered in the single dose study and daily doses of 30 mg/m2, 90 mg/m2 and 210 mg/m2 were administered q 8h in the multi-dose study. After rapid absorption with peak levels occurring on average between 2 to 3 hours following administration, sotalol was eliminated with a mean half life of 9.5 hours. Steady-state was reached after 1 to 2 days. The average peak to trough concentration ratio was 2. BSA was the most important covariate and more relevant than age for the pharmacokinetics of sotalol. The smallest children (BSA< 0.33m2) exhibited a greater drug exposure (+59%) than the larger children who showed a uniform drug concentration profile. The intersubject variation for oral clearance was 22%.
-
INDICATIONS AND USAGE
Sotalol hydrochloride tablets USP are indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that in the judgment of the physician are life-threatening. Because of the proarrhythmic effects of sotalol hydrochloride tablets (see WARNINGS), including a 1.5% to 2% rate of torsade de pointes or new VT/VF in patients with either NSVT or supraventricular arrhythmias, its use in patients with less severe arrhythmias, even if the patients are symptomatic, is generally not recommended. Treatment of patients with asymptomatic ventricular premature contractions should be avoided.
Initiation of sotalol hydrochloride tablet USP treatment or increasing doses, as with other antiarrhythmic agents used to treat life-threatening arrhythmias, should be carried out in the hospital. The response to treatment should then be evaluated by a suitable method (e.g., PES or Holter monitoring) prior to continuing the patient on chronic therapy. Various approaches have been used to determine the response to antiarrhythmic therapy, including sotalol hydrochloride tablets USP.
In the ESVEM Trial, response by Holter monitoring was tentatively defined as 100% suppression of ventricular tachycardia, 90% suppression of non-sustained VT, 80% suppression of paired VPCs and 75% suppression of total VPCs in patients who had at least 10 VPCs/hour at baseline; this tentative response was confirmed if VT lasting 5 or more beats was not observed during treadmill exercise testing using a standard Bruce protocol. The PES protocol utilized a maximum of three extrastimuli at three pacing cycle lengths and two right ventricular pacing sites. Response to PES was defined as prevention of induction of the following: 1) monomorphic VT lasting over 15 seconds; 2) non-sustained polymorphic VT containing more than 15 beats of monomorphic VT in patients with a history of monomorphic VT; 3) polymorphic VT or VF greater than 15 beats in patients with VF or a history of aborted sudden death without monomorphic VT; and 4) two episodes of polymorphic VT or VF of greater than 15 beats in a patient presenting with monomorphic VT. Sustained VT or NSVT producing hypotension during the final treadmill test was considered a drug failure.
In a multicenter open-label long-term study of sotalol hydrochloride tablets USP in patients with life-threatening ventricular arrhythmias which had proven refractory to other antiarrhythmic medications, response by Holter monitoring was defined as in ESVEM. Response by PES was defined as non-inducibility of sustained VT by at least double extrastimuli delivered at a pacing cycle length of 400 msec. Overall survival and arrhythmia recurrence rates in this study were similar to those seen in ESVEM, although there was no comparative group to allow a definitive assessment of outcome.
Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias.
Sotalol is also indicated for the maintenance of normal sinus rhythm [delay in time to recurrence of atrial fibrillation/atrial flutter (AFIB/AFL)] in patients with symptomatic AFIB/AFL who are currently in sinus rhythm and is marketed under the brand name BETAPACE AFTM. Sotalol hydrochloride tablets USP are not approved for the AFIB/AFL indication and should not be substituted for BETAPACE AFTM because only BETAPACE AFTM is distributed with a patient package insert that is appropriate for patients with AFIB/AFL.
-
CONTRAINDICATIONS
Sotalol hydrochloride tablets are contraindicated in patients with bronchial asthma, sinus bradycardia, second and third degree AV block, unless a functioning pacemaker is present, congenital or acquired long QT syndromes, cardiogenic shock, uncontrolled congestive heart failure and previous evidence of hypersensitivity to sotalol hydrochloride tablets.
-
WARNINGS
Mortality
The National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial I (CAST I) was a long-term, multi-center, double-blind study in patients with asymptomatic, non-life-threatening ventricular arrhythmias, 1 to 103 weeks after acute myocardial infarction. Patients in CAST I were randomized to receive placebo or individually optimized doses of encainide, flecainide or moricizine. The Cardiac Arrhythmia Suppression Trial II (CAST II) was similar, except that the recruited patients had had their index infarction 4 to 90 days before randomization, patients with left ventricular ejection fractions greater than 40% were not admitted and the randomized regimens were limited to placebo and moricizine.
CAST I was discontinued after an average time-on-treatment of 10 months and CAST II was discontinued after an average time-on-treatment of 18 months. As compared to placebo treatment, all three active therapies were associated with increases in short-term (14-day) mortality and encainide and flecainide were associated with significant increases in longer-term mortality as well. The longer-term mortality rate associated with moricizine treatment could not be statistically distinguished from that associated with placebo.
The applicability of these results to other populations (e.g., those without recent myocardial infarction) and to other than Class I antiarrhythmic agents is uncertain. Sotalol hydrochloride tablets are devoid of Class I effects and in a large (n=1,456) controlled trial in patients with a recent myocardial infarction, who did not necessarily have ventricular arrhythmias, sotalol hydrochloride tablets did not produce increased mortality at doses up to 320 mg/day (see CLINICAL PHARMACOLOGY, Clinical Actions). On the other hand, in the large post-infarction study using a non-titrated initial dose of 320 mg once daily and in a second small randomized trial in high-risk post-infarction patients treated with high doses (320 mg BID), there have been suggestions of an excess of early sudden deaths.
Proarrhythmia
Like other antiarrhythmic agents, sotalol hydrochloride tablets can provoke new or worsened ventricular arrhythmias in some patients, including sustained ventricular tachycardia or ventricular fibrillation, with potentially fatal consequences. Because of its effect on cardiac repolarization (QTc interval prolongation), torsade de pointes, a polymorphic ventricular tachycardia with prolongation of the QT interval and a shifting electrical axis is the most common form of proarrhythmia associated with sotalol hydrochloride tablets, occurring in about 4% of high risk (history of sustained VT/VF) patients. The risk of torsade de pointes progressively increases with prolongation of the QT interval and is worsened also by reduction in heart rate and reduction in serum potassium (see WARNINGS, Electrolyte Disturbances).
Because of the variable temporal recurrence of arrhythmias, it is not always possible to distinguish between a new or aggravated arrhythmic event and the patient's underlying rhythm disorder. (Note, however, that torsade de pointes is usually a drug-induced arrhythmia in people with an initially normal QTc). Thus, the incidence of drug-related events cannot be precisely determined, so that the occurrence rates provided must be considered approximations. Note also that drug-induced arrhythmias may often not be identified, particularly if they occur long after starting the drug, due to less frequent monitoring. It is clear from the NIH-sponsored CAST (see WARNINGS, Mortality) that some antiarrhythmic drugs can cause increased sudden death mortality, presumably due to new arrhythmias or asystole, that do not appear early in treatment but that represent a sustained increased risk.
Overall in clinical trials with sotalol, 4.3% of 3,257 patients experienced a new or worsened ventricular arrhythmia. Of this 4.3%, there was new or worsened sustained ventricular tachycardia in approximately 1% of patients and torsade de pointes in 2.4%. Additionally, in approximately 1% of patients, deaths were considered possibly drug-related; such cases, although difficult to evaluate, may have been associated with proarrhythmic events.
In patients with a history of sustained ventricular tachycardia, the incidence of torsade de pointes was 4% and worsened VT in about 1%; in patients with other, less serious, ventricular arrhythmias and supraventricular arrhythmias, the incidence of torsade de pointes was 1% and 1.4%, respectively.
Torsade de pointes arrhythmias were dose related, as is the prolongation of QT (QTc) interval, as shown in the table below.
Percent Incidence of Torsade de Pointes and Mean QTc Interval by Dose for Patients with Sustained VT/VF Daily Dose (mg) Incidence of Torsade de Pointes Mean QTc* (msec) 80 0 (69)† 463 (17) 160 0.5 (832) 467 (181) 320 1.6 (835) 473 (344) 480 4.4 (459) 483 (234) 640 3.7 (324) 490 (185) >640 5.8 (103) 512 (62) In addition to dose and presence of sustained VT, other risk factors for torsade de pointes were gender (females had a higher incidence), excessive prolongation of the QTc interval (see table below) and history of cardiomegaly or congestive heart failure. Patients with sustained ventricular tachycardia and a history of congestive heart failure appear to have the highest risk for serious proarrhythmia (7%). Of the patients experiencing torsade de pointes, approximately two-thirds spontaneously reverted to their baseline rhythm. The others were either converted electrically (D/C cardioversion or overdrive pacing) or treated with other drugs (see OVERDOSAGE). It is not possible to determine whether some sudden deaths represented episodes of torsade de pointes, but in some instances sudden death did follow a documented episode of torsade de pointes. Although sotalol hydrochloride tablet therapy was discontinued in most patients experiencing torsade de pointes, 17% were continued on a lower dose.
Nonetheless, sotalol hydrochloride tablets should be used with particular caution if the QTc is greater than 500 msec on-therapy and serious consideration should be given to reducing the dose or discontinuing therapy when the QTc exceeds 550 msec. Due to the multiple risk factors associated with torsade de pointes, however, caution should be exercised regardless of the QTc interval. The table below relates the incidence of torsade de pointes to on-therapy QTc and change in QTc from baseline. It should be noted, however, that the highest on-therapy QTc was in many cases the one obtained at the time of the torsade de pointes event, so that the table overstates the predictive value of a high QTc.
Relationship Between QTc Interval Prolongation and Torsade de Pointes On-Therapy QTc Interval (msec) Incidence of Torsade de Pointes Change in QTc Interval From Baseline (msec) Incidence of Torsade de Pointes <500 1.3% (1,787) <65 1.6% (1,516) 500-525 3.4% (236) 65-80 3.2% (158) 525-550 5.6% (125) 80-100 4.1% (146) >550 10.8% (157) 100-130 5.2% (115) >130 7.1% (99) () Number of patients assessed
Proarrhythmic events must be anticipated not only on initiating therapy, but with every upward dose adjustment. Proarrhythmic events most often occur within 7 days of initiating therapy or of an increase in dose; 75% of serious proarrhythmias (torsade de pointes and worsened VT) occurred within 7 days of initiating sotalol hydrochloride tablet therapy, while 60% of such events occurred within 3 days of initiation or a dosage change. Initiating therapy at 80 mg BID with gradual upward dose titration and appropriate evaluations for efficacy (e.g., PES or Holter) and safety (e.g., QT interval, heart rate and electrolytes) prior to dose escalation, should reduce the risk of proarrhythmia. Avoiding excessive accumulation of sotalol in patients with diminished renal function, by appropriate dose reduction, should also reduce the risk of proarrhythmia (see DOSAGE AND ADMINISTRATION).
Congestive Heart Failure
Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure and beta-blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure. In patients who have congestive heart failure controlled by digitalis and/or diuretics, sotalol hydrochloride tablets should be administered cautiously. Both digitalis and sotalol slow AV conduction. As with all beta-blockers, caution is advised when initiating therapy in patients with any evidence of left ventricular dysfunction. In pre-marketing studies, new or worsened congestive heart failure (CHF) occurred in 3.3% (n=3,257) of patients and led to discontinuation in approximately 1% of patients receiving sotalol. The incidence was higher in patients presenting with sustained ventricular tachycardia/fibrillation (4.6%, n=1,363) or a prior history of heart failure (7.3%, n=696). Based on a life-table analysis, the one-year incidence of new or worsened CHF was 3% in patients without a prior history and 10% in patients with a prior history of CHF. NYHA Classification was also closely associated to the incidence of new or worsened heart failure while receiving sotalol (1.8% in 1,395 Class I patients, 4.9% in 1,254 Class II patients and 6.1% in 278 Class III or IV patients).
Electrolyte Disturbances
Sotalol should not be used in patients with hypokalemia or hypomagnesemia prior to correction of imbalance, as these conditions can exaggerate the degree of QT prolongation and increase the potential for torsade de pointes. Special attention should be given to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or patients receiving concomitant diuretic drugs.
Conduction Disturbances
Excessive prolongation of the QT interval (>550 msec) can promote serious arrhythmias and should be avoided (see WARNINGS, Proarrhythmia). Sinus bradycardia (heart rate less than 50 bpm) occurred in 13% of patients receiving sotalol hydrochloride tablets in clinical trials and led to discontinuation in about 3% of patients. Bradycardia itself increases the risk of torsade de pointes. Sinus pause, sinus arrest and sinus node dysfunction occur in less than 1% of patients. The incidence of 2nd- or 3rd-degree AV block is approximately 1%.
Recent Acute MI
Sotalol hydrochloride tablets can be used safely and effectively in the long-term treatment of life-threatening ventricular arrhythmias following a myocardial infarction. However, experience in the use of sotalol hydrochloride tablets to treat cardiac arrhythmias in the early phase of recovery from acute MI is limited and at least at high initial doses is not reassuring (see WARNINGS, Mortality). In the first 2 weeks post-MI caution is advised and careful dose titration is especially important, particularly in patients with markedly impaired ventricular function.
The following warnings are related to the beta-blocking activity of sotalol hydrochloride tablets.
Abrupt Withdrawal
Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy. Occasional cases of exacerbation of angina pectoris, arrhythmias and, in some cases, myocardial infarction have been reported after abrupt discontinuation of beta-blocker therapy. Therefore, it is prudent when discontinuing chronically administered sotalol hydrochloride tablets, particularly in patients with ischemic heart disease, to carefully monitor the patient and consider the temporary use of an alternate beta-blocker if appropriate. If possible, the dosage of sotalol hydrochloride tablets should be gradually reduced over a period of one to two weeks. If angina or acute coronary insufficiency develops, appropriate therapy should be instituted promptly. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized in patients receiving sotalol hydrochloride tablets, abrupt discontinuation in patients with arrhythmias may unmask latent coronary insufficiency.
Non-Allergic Bronchospasm (e.g., chronic bronchitis and emphysema)
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD IN GENERAL NOT RECEIVE BETA-BLOCKERS.
It is prudent, if sotalol hydrochloride tablets are to be administered, to use the smallest effective dose, so that inhibition of bronchodilation produced by endogenous or exogenous catecholamine stimulation of beta2 receptors may be minimized.
Anaphylaxis
While taking beta-blockers, patients with a history of anaphylactic reaction to a variety of allergens may have a more severe reaction on repeated challenge, either accidental, diagnostic or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat the allergic reaction.
Anesthesia
The management of patients undergoing major surgery who are being treated with beta-blockers is controversial. Protracted severe hypotension and difficulty in restoring and maintaining normal cardiac rhythm after anesthesia have been reported in patients receiving beta-blockers.
Diabetes
In patients with diabetes (especially labile diabetes) or with a history of episodes of spontaneous hypoglycemia, sotalol hydrochloride tablets should be given with caution since beta-blockade may mask some important premonitory signs of acute hypoglycemia; e.g., tachycardia.
Sick Sinus Syndrome
Sotalol hydrochloride tablets should be used only with extreme caution in patients with sick sinus syndrome associated with symptomatic arrhythmias, because it may cause sinus bradycardia, sinus pauses or sinus arrest.
Thyrotoxicosis
Beta-blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-blockade which might be followed by an exacerbation of symptoms of hyperthyroidism, including thyroid storm.
-
PRECAUTIONS
Renal Impairment
Sotalol hydrochloride tablets are mainly eliminated via the kidneys through glomerular filtration and to a small degree by tubular secretion. There is a direct relationship between renal function, as measured by serum creatinine or creatinine clearance and the elimination rate of sotalol hydrochloride tablets. Guidance for dosing in conditions of renal impairment can be found under DOSAGE AND ADMINISTRATION.
Drug Interactions
Drugs undergoing CYP450 metabolism
Sotalol is primarily eliminated by renal excretion; therefore, drugs that are metabolized by CYP450 are not expected to alter the pharmacokinetics of sotalol. Sotalol is not expected to inhibit or induce any CYP450 enzymes; therefore, it is not expected to alter the PK of drugs that are metabolized by these enzymes.
Antiarrhythmics
Class 1a antiarrhythmic drugs, such as disopyramide, quinidine and procainamide and other Class III drugs (e.g., amiodarone) are not recommended as concomitant therapy with sotalol hydrochloride tablets because of their potential to prolong refractoriness (see WARNINGS). There is only limited experience with the concomitant use of Class 1b or 1c antiarrhythmics. Additive Class II effects would also be anticipated with the use of other beta-blocking agents concomitantly with sotalol hydrochloride tablets.
Digoxin
Single and multiple doses of sotalol hydrochloride tablets do not substantially affect serum digoxin levels. Proarrhythmic events were more common in sotalol hydrochloride tablet treated patients also receiving digoxin; it is not clear whether this represents an interaction or is related to the presence of CHF, a known risk factor for proarrhythmia, in the patients receiving digoxin.
Calcium-blocking drugs
Sotalol hydrochloride tablets should be administered with caution in conjunction with calcium-blocking drugs because of possible additive effects on atrioventricular conduction or ventricular function. Additionally, concomitant use of these drugs may have additive effects on blood pressure, possibly leading to hypotension.
Catecholamine-depleting agents
Concomitant use of catecholamine-depleting drugs, such as reserpine and guanethidine, with a beta-blocker may produce an excessive reduction of resting sympathetic nervous tone. Patients treated with sotalol hydrochloride tablets plus a catecholamine depletor should therefore be closely monitored for evidence of hypotension and/or marked bradycardia which may produce syncope.
Insulin and oral antidiabetics
Hyperglycemia may occur and the dosage of insulin or antidiabetic drugs may require adjustment. Symptoms of hypoglycemia may be masked.
Beta-2-receptor stimulants
Beta-agonists such as salbutamol, terbutaline and isoprenaline may have to be administered in increased dosages when used concomitantly with sotalol hydrochloride tablets.
Clonidine
Beta-blocking drugs may potentiate the rebound hypertension sometimes observed after discontinuation of clonidine; therefore, caution is advised when discontinuing clonidine in patients receiving sotalol hydrochloride tablets.
Antacids
Administration of sotalol hydrochloride tablets within 2 hours of antacids containing aluminum oxide and magnesium hydroxide should be avoided because it may result in a reduction in Cmax and AUC of 26% and 20%, respectively and consequently in a 25% reduction in the bradycardic effect at rest. Administration of the antacid two hours after sotalol hydrochloride tablets has no effect on the pharmacokinetics or pharmacodynamics of sotalol.
Drugs prolonging the QT interval
Sotalol hydrochloride tablets should be administered with caution in conjunction with other drugs known to prolong the QT interval such as Class I and Class III antiarrhythmic agents, phenothiazines, tricyclic antidepressants, astemizole, bepridil, certain oral macrolides and certain quinolone antibiotics (see WARNINGS).
Drug/Laboratory Test Interactions
The presence of sotalol in the urine may result in falsely elevated levels of urinary metanephrine when measured by fluorimetric or photometric methods. In screening patients suspected of having a pheochromocytoma and being treated with sotalol, a specific method, such as a high performance liquid chromatographic assay with solid phase extraction (e.g., J. Chromatogr. 385:241, 1987) should be employed in determining levels of catecholamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential was observed in rats during a 24 month study at 137 mg/kg/day to 275 mg/kg/day (approximately 30 times the maximum recommended human oral dose (MRHD) as mg/kg or 5 times the MRHD as mg/m2) or in mice, during a 24-month study at 4,141 mg/kg/day to 7,122 mg/kg/day (approximately 450 to 750 times the MRHD as mg/kg or 36 to 63 times the MRHD as mg/m2).
Sotalol has not been evaluated in any specific assay of mutagenicity or clastogenicity.
No significant reduction in fertility occurred in rats at oral doses of 1,000 mg/kg/day (approximately 100 times the MRHD as mg/kg or 9 times the MRHD as mg/m2) prior to mating, except for a small reduction in the number of offspring per litter.
Pregnancy
Category B
Reproduction studies in rats and rabbits during organogenesis at 100 and 22 times the MRHD as mg/kg (9 and 7 times the MRHD as mg/m2), respectively, did not reveal any teratogenic potential associated with sotalol hydrochloride. In rabbits, a high dose of sotalol hydrochloride (160 mg/kg/day) at 16 times the MRHD as mg/kg (6 times the MRHD as mg/m2) produced a slight increase in fetal death likely due to maternal toxicity. Eight times the maximum dose (80 mg/kg/day or 3 times the MRHD as mg/m2) did not result in an increased incidence of fetal deaths. In rats, 1000 mg/kg/day sotalol hydrochloride, 100 times the MRHD (18 times the MRHD as mg/m2), increased the number of early resorptions, while at 14 times the maximum dose (2.5 times the MRHD as mg/m2), no increase in early resorptions was noted. However, animal reproduction studies are not always predictive of human response.
Although there are no adequate and well-controlled studies in pregnant women, sotalol hydrochloride has been shown to cross the placenta and is found in amniotic fluid. There has been a report of subnormal birth weight with sotalol hydrochloride tablets. Therefore, sotalol hydrochloride tablets should be used during pregnancy only if the potential benefit outweighs the potential risk.
Nursing Mothers
Sotalol is excreted in the milk of laboratory animals and has been reported to be present in human milk. Because of the potential for adverse reactions in nursing infants from sotalol hydrochloride tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of sotalol hydrochloride tablets in children have not been established. However, the Class III electrophysiologic and beta-blocking effects, the pharmacokinetics and the relationship between the effects (QTc interval and resting heart rate) and drug concentrations have been evaluated in children aged between 3 days and 12 years old (see CLINICAL PHARMACOLOGY).
-
ADVERSE REACTIONS
During pre-marketing trials, 3,186 patients with cardiac arrhythmias (1,363 with sustained ventricular tachycardia) received oral sotalol hydrochloride tablets, of whom 2,451 received the drug for at least two weeks. The most important adverse effects are torsade de pointes and other serious new ventricular arrhythmias (see WARNINGS), occurring at rates of almost 4% and 1%, respectively, in the VT/VF population. Overall, discontinuation because of unacceptable side-effects was necessary in 17% of all patients in clinical trials and in 13% of patients treated for at least two weeks. The most common adverse reactions leading to discontinuation of sotalol hydrochloride tablets are as follows: fatigue 4%, bradycardia (less than 50 bpm) 3%, dyspnea 3%, proarrhythmia 3%, asthenia 2% and dizziness 2%. Occasional reports of elevated serum liver enzymes have occurred with sotalol hydrochloride tablet therapy but no cause and effect relationship has been established. One case of peripheral neuropathy which resolved on discontinuation of sotalol hydrochloride tablets and recurred when the patient was rechallenged with the drug was reported in an early dose tolerance study. Elevated blood glucose levels and increased insulin requirements can occur in diabetic patients.
The following table lists as a function of dosage the most common (incidence of 2% or greater) adverse events, regardless of relationship to therapy and the percent of patients discontinued due to the event, as collected from clinical trials involving 1,292 patients with sustained VT/VF.
Incidence (%) of Adverse Events and Discontinuations - *
- Because patients are counted at each dose level tested, the Any Dose column cannot be determined by adding across the doses.
DAILY DOSE Body System 160 mg
(n=832)240 mg
(n=263)320 mg
(n=835)480 mg
(n=459)640 mg
(n=324)Any Dose*
(n=1,292)% Patients Discontinued
(n=1,292)Body as a Whole infection 1 2 2 2 3 4 <1 fever 1 2 3 2 2 4 <1 localized pain 1 1 2 2 2 3 <1 Cardiovascular dyspnea 5 8 11 15 15 21 2 bradycardia 8 8 9 7 5 16 2 chest pain 4 3 10 10 14 16 <1 palpitation 3 3 8 9 12 14 <1 edema 2 2 5 3 5 8 1 ECG abnormal 4 2 4 2 2 7 1 hypotension 3 4 3 2 3 6 2 proarrhythmia <1 <1 2 4 5 5 3 syncope 1 1 3 2 5 5 1 heart failure 2 3 2 2 2 5 1 presyncope 1 2 2 4 3 4 <1 peripheral vascular disorder 1 2 1 1 2 3 <1 cardiovascular disorder 1 <1 2 2 2 3 <1 vasodilation 1 <1 1 2 1 3 <1 AICD discharge <1 2 2 2 2 3 <1 hypertension <1 1 1 1 2 2 <1 Nervous fatigue 5 8 12 12 13 20 2 dizziness 7 6 11 11 14 20 1 asthenia 4 5 7 8 10 13 1 light-headed 4 3 6 6 9 12 1 headache 3 2 4 4 4 8 <1 sleep problem 1 1 5 5 6 8 <1 perspiration 1 2 3 4 5 6 <1 altered consciousness 2 3 1 2 3 4 <1 depression 1 2 2 2 3 4 <1 paresthesia 1 1 2 3 2 4 <1 anxiety 2 2 2 3 2 4 <1 mood change <1 <1 1 3 2 3 <1 appetite disorder 1 2 2 1 3 3 <1 stroke <1 <1 1 1 <1 1 <1 Digestive nausea/vomiting 5 4 4 6 6 10 1 diarrhea 2 3 3 3 5 7 <1 dyspepsia 2 3 3 3 3 6 <1 abdominal pain <1 <1 2 2 2 3 <1 colon problem 2 1 1 <1 2 3 <1 flatulence 1 <1 1 1 2 2 <1 Respiratory pulmonary problem 3 3 5 3 4 8 <1 upper respiratory tract problem 1 1 3 4 3 5 <1 asthma 1 <1 1 1 1 2 <1 Urogenital genitourinary disorder 1 0 1 1 2 3 <1 sexual dysfunction <1 1 1 1 3 2 <1 Metabolic abnormal lab value 1 2 3 2 1 4 <1 weight change 1 1 1 <1 2 2 <1 Musculoskeletal extremity pain 2 2 4 5 3 7 <1 back pain 1 <1 2 2 2 3 <1 Skin and Appendages rash 2 3 2 3 4 5 <1 Hematologic bleeding 1 <1 1 <1 2 2 <1 Special Senses visual problem 1 1 2 4 5 5 <1 In an unblinded multicenter trial of 25 patients with SVT and/or VT receiving daily doses of 30 mg/m2, 90 mg/m2 and 210 mg/m2 with dosing every 8 hours for a total of 9 doses, no torsade de pointes or other serious new arrhythmias were observed. One (1) patient, receiving 30 mg/m2 daily, was discontinued because of increased frequency of sinus pauses/bradycardia. Additional cardiovascular AEs were seen at the 90 mg/m2 and 210 mg/m2 daily dose levels. They included QT prolongations (2 patients), sinus pauses/bradycardia (1 patient), increased severity of atrial flutter and reported chest pain (1 patient). Values for QTc ≥ 525 msec were seen in 2 patients at the 210 mg/m2 daily dose level. Serious adverse events including death, torsade de pointes, other proarrhythmias, high-degree A-V blocks and bradycardia have been reported in infants and/or children.
Potential Adverse Effects
Foreign marketing experience with sotalol hydrochloride shows an adverse experience profile similar to that described above from clinical trials. Voluntary reports since introduction include rare reports (less than one report per 10,000 patients) of: emotional lability, slightly clouded sensorium, incoordination, vertigo, paralysis, thrombocytopenia, eosinophilia, leukopenia, photosensitivity reaction, fever, pulmonary edema, hyperlipidemia, myalgia, pruritus, alopecia.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been associated with sotalol hydrochloride tablets during investigational use and foreign marketing experience.
-
OVERDOSAGE
Intentional or accidental overdosage with sotalol hydrochloride has rarely resulted in death.
Symptoms and Treatment of Overdosage
The most common signs to be expected are bradycardia, congestive heart failure, hypotension, bronchospasm and hypoglycemia. In cases of massive intentional overdosage (2 grams to 16 grams) of sotalol hydrochloride tablets the following clinical findings were seen: hypotension, bradycardia, cardiac asystole, prolongation of QT interval, torsade de pointes, ventricular tachycardia and premature ventricular complexes. If overdosage occurs, therapy with sotalol hydrochloride tablets should be discontinued and the patient observed closely. Because of the lack of protein binding, hemodialysis is useful for reducing sotalol plasma concentrations. Patients should be carefully observed until QT intervals are normalized and the heart rate returns to levels >50 bpm. The occurrence of hypotension following an overdose may be associated with an initial slow drug elimination phase (half life of 30 hours) thought to be due to a temporary reduction of renal function caused by the hypotension. In addition, if required, the following therapeutic measures are suggested:
Bradycardia or Cardiac Asystole: Atropine, another anticholinergic drug, a beta-adrenergic agonist or transvenous cardiac pacing.
Heart Block:(second and third degree) transvenous cardiac pacemaker.
Hypotension:(depending on associated factors) epinephrine rather than isoproterenol or norepinephrine may be useful.
Bronchospasm:Aminophylline or aerosol beta-2-receptor stimulant.
torsade de pointes:DC cardioversion, transvenous cardiac pacing, epinephrine, magnesium sulfate.
-
DOSAGE AND ADMINISTRATION
As with other antiarrhythmic agents, sotalol hydrochloride tablets should be initiated and doses increased in a hospital with facilities for cardiac rhythm monitoring and assessment (see INDICATIONS AND USAGE). Sotalol hydrochloride tablets should be administered only after appropriate clinical assessment (see INDICATIONS AND USAGE) and the dosage of sotalol hydrochloride tablets must be individualized for each patient on the basis of therapeutic response and tolerance. Proarrhythmic events can occur not only at initiation of therapy, but also with each upward dosage adjustment.
Adults
Dosage of sotalol hydrochloride tablets should be adjusted gradually, allowing 3 days between dosing increments in order to attain steady-state plasma concentrations and to allow monitoring of QT intervals. Graded dose adjustment will help prevent the usage of doses which are higher than necessary to control the arrhythmia. The recommended initial dose is 80 mg twice daily. This dose may be increased, if necessary, after appropriate evaluation to 240 mg/day or 320 mg/day (120 mg to 160 mg twice daily). In most patients, a therapeutic response is obtained at a total daily dose of 160 mg/day to 320 mg/day, given in two or three divided doses. Some patients with life-threatening refractory ventricular arrhythmias may require doses as high as 480 mg/day to 640 mg/day; however, these doses should only be prescribed when the potential benefit outweighs the increased risk of adverse events, in particular proarrhythmia. Because of the long terminal elimination half-life of sotalol hydrochloride tablets, dosing on more than a BID regimen is usually not necessary.
Children
As in adults the following precautionary measures should be considered when initiating sotalol treatment in children: initiation of treatment in the hospital after appropriate clinical assessment; individualized regimen as appropriate; gradual increase of doses if required; careful assessment of therapeutic response and tolerability; and frequent monitoring of the QTc interval and heart rate.
For children aged about 2 years and greater: With normal renal function, doses normalized for body surface area are appropriate for both initial and incremental dosing. Since the Class III potency in children (see CLINICAL PHARMACOLOGY) is not very different from that in adults, reaching plasma concentrations that occur within the adult dose range is an appropriate guide. From pediatric pharmacokinetic data the following is recommended.
For initiation of treatment, 30 mg/m2 three times a day (90 mg/m2 total daily dose) is approximately equivalent to the initial 160 mg total daily dose for adults. Subsequent titration to a maximum of 60 mg/m2 (approximately equivalent to the 360 mg total daily dose for adults) can then occur. Titration should be guided by clinical response, heart rate and QTc, with increased dosing being preferably carried out in-hospital. At least 36 hours should be allowed between dose increments to attain steady-state plasma concentrations of sotalol in patients with age-adjusted normal renal function.
For children aged about 2 years or younger: The above pediatric dosage should be reduced by a factor that depends heavily upon age, as shown in the following graph, age plotted on a logarithmic scale in months.
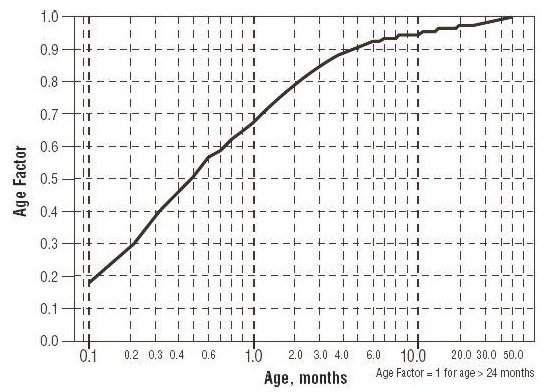
For a child aged 20 months, the dosing suggested for children with normal renal function aged 2 years or greater should be multiplied by about 0.97; the initial starting dose would be (30 X 0.97)=29.1 mg/m2, administered three times daily. For a child aged 1 month, the starting dose should be multiplied by 0.68; the initial starting dose would be (30 X 0.68)= 20 mg/m2, administered three times daily. For a child aged about 1 week, the initial starting dose should be multiplied by 0.3; the starting dose would be (30 X 0.3)=9 mg/m2. Similar calculations should be made for increased doses as titration proceeds. Since the half-life of sotalol decreases with decreasing age (below about 2 years), time to steady-state will also increase. Thus, in neonates the time to steady-state may be as long as a week or longer.
In all children, individualization of dosage is required. As in adults, sotalol hydrochloride tablets should be used with particular caution in children if the QTc is greater than 500 msec on therapy and serious consideration should be given to reducing the dose or discontinuing therapy when QTc exceeds 550 msec.
Dosage In Renal Impairment
Adults: Because sotalol is excreted predominantly in urine and its terminal elimination half-life is prolonged in conditions of renal impairment, the dosing interval (time between divided doses) of sotalol should be modified (when creatinine clearance is lower than 60 mL/min) according to the following table.
Creatinine Clearance Dosing* Interval mL/min (hours) >60 12 30 - 59 24 10 - 29 36 - 48 <10 Dose should be individualized *The initial dose of 80 mg and subsequent doses should be administered at these intervals. See following paragraph for dosage escalations.
Since the terminal elimination half-life of sotalol hydrochloride tablets has increased in patients with renal impairment, a longer duration of dosing is required to reach steady-state. Dose escalations in renal impairment should be done after administration of at least 5 to 6 doses at appropriate intervals (see table above).
Extreme caution should be exercised in the use of sotalol in patients with renal failure undergoing hemodialysis. The half-life of sotalol is prolonged (up to 69 hours) in anuric patients. Sotalol, however, can be partly removed by dialysis with subsequent partial rebound in concentrations when dialysis is completed. Both safety (heart rate, QT interval) and efficacy (arrhythmia control) must be closely monitored.
Children: The use of sotalol hydrochloride tablets in children with renal impairment has not been investigated. Sotalol elimination is predominantly via the kidney in the unchanged form. Use of sotalol in any age group with decreased renal function should be at lower doses or at increased intervals between doses. Monitoring of heart rate and QTc is more important and it will take much longer to reach steady-state with any dose and/or frequency of administration.
Transfer to Sotalol Hydrochloride Tablets
Before starting sotalol, previous antiarrhythmic therapy should generally be withdrawn under careful monitoring for a minimum of 2 to 3 plasma half-lives if the patient’s clinical condition permits (see PRECAUTIONS, Drug Interactions). Treatment has been initiated in some patients receiving I.V. lidocaine without ill effect. After discontinuation of amiodarone, sotalol should not be initiated until the QT interval is normalized (see WARNINGS).
Preparation of Extemporaneous Oral Solution
Sotalol Hydrochloride Syrup 5 mg/mL can be compounded using Simple Syrup containing 0.1% sodium benzoate (Syrup, NF) available from Humco Laboratories as follows:
- Measure 120 mL of Simple Syrup.
- Transfer the syrup to a 6-ounce amber plastic (polyethylene terephthalate [PET]) prescription bottle. NOTE: An oversized bottle is used to allow for a headspace, so that there will be more effective mixing during shaking of the bottle.
- Add five (5) sotalol hydrochloride 120 mg tablets to the bottle. These tablets are added intact; it is not necessary to crush the tablets. NOTE: The addition of the tablets can also be done first. The tablets can also be crushed if preferred. If the tablets are crushed, care should be taken to transfer the entire quantity of tablet powder into the bottle containing the syrup.
- Shake the bottle to wet the entire surface of the tablets. If the tablets have been crushed, shake the bottle until the endpoint is achieved.
- Allow the tablets to hydrate for at least two hours.
- After at least two hours have elapsed, shake the bottle intermittently over the course of at least another two hours until the tablets are completely disintegrated. NOTE: The tablets can be allowed to hydrate overnight to simplify the disintegration process.
The endpoint is achieved when a dispersion of fine particles in the syrup is obtained.
This compounding procedure results in a solution containing 5 mg/mL of sotalol HCl. The fine solid particles are the water-insoluble inactive ingredients of the tablets.
This extemporaneously prepared oral solution of sotalol HCl (with suspended inactive particles) must be shaken well prior to administration. This is to ensure that the amount of inactive solid particles per dose remains constant throughout the duration of use.
Stability studies indicate that the suspension is stable for three months when stored at controlled room temperature (15° to 30°C/59° to 86°F) and ambient humidity.
Transfer to BETAPACETM AF from Sotalol Hydrochloride Tablets
Patients with a history of symptomatic AFIB/AFL who are currently receiving sotalol hydrochloride tablets for the maintenance of normal sinus rhythm should be transferred to BETAPACE AFTM because of the significant differences in labeling (i.e., patient package insert for BETAPACE AFTM, dosing administration and safety information).
-
HOW SUPPLIED
Sotalol Hydrochloride Tablets USP for oral administration are available as follows:
80 mg: Blue, capsule shaped tablets, debossed “E 171” on one side and bisected on the reverse side and supplied as:
NDC 54868-4435-0 bottles of 30
NDC 54868-4435-1 bottles of 60
NDC 54868-4435-3 bottles of 90
NDC 54868-4435-2 bottles of 100
120 mg: Blue, capsule shaped tablets, debossed “E 170” on one side and bisected on the reverse side and supplied as:
NDC 54868-5614-1 bottles of 20
NDC 54868-5614-0 bottles of 60
160 mg: Blue, capsule shaped tablets, debossed “E 177” on one side and bisected on the reverse side and supplied as:
NDC 54868-5549-1 bottles of 20
NDC 54868-5549-0 bottles of 60
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Sandoz Inc.
Princeton, NJ 08540
OS7554
REV. 02/10
MF0171REV02/10
MG #18481
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
- Sotalol Hydrochloride Tablets USP, 80 mg Tablets - Label
- Sotalol Hydrochloride Tablets USP, 120 mg Tablets - Label
- Sotalol Hydrochloride Tablets USP, 160 mg Tablets - Label
-
INGREDIENTS AND APPEARANCE
SOTALOL HYDROCHLORIDE
sotalol hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-4435(NDC:0185-0171) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTALOL HYDROCHLORIDE (UNII: HEC37C70XX) (SOTALOL - UNII:A6D97U294I) SOTALOL HYDROCHLORIDE 80 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color BLUE ((Light)) Score 2 pieces Shape CAPSULE Size 12mm Flavor Imprint Code E171 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-4435-0 30 in 1 BOTTLE 2 NDC:54868-4435-1 60 in 1 BOTTLE 3 NDC:54868-4435-2 100 in 1 BOTTLE 4 NDC:54868-4435-3 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075366 12/04/2000 SOTALOL HYDROCHLORIDE
sotalol hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5614(NDC:0185-0170) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTALOL HYDROCHLORIDE (UNII: HEC37C70XX) (SOTALOL - UNII:A6D97U294I) SOTALOL HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color BLUE ((Light)) Score 2 pieces Shape CAPSULE Size 14mm Flavor Imprint Code E170 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5614-0 60 in 1 BOTTLE 2 NDC:54868-5614-1 20 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075366 04/08/2009 SOTALOL HYDROCHLORIDE
sotalol hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5549(NDC:0185-0177) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTALOL HYDROCHLORIDE (UNII: HEC37C70XX) (SOTALOL - UNII:A6D97U294I) SOTALOL HYDROCHLORIDE 160 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color BLUE ((Light)) Score 2 pieces Shape CAPSULE Size 15mm Flavor Imprint Code E177 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5549-0 60 in 1 BOTTLE 2 NDC:54868-5549-1 20 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075366 03/14/2006 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel, repack






