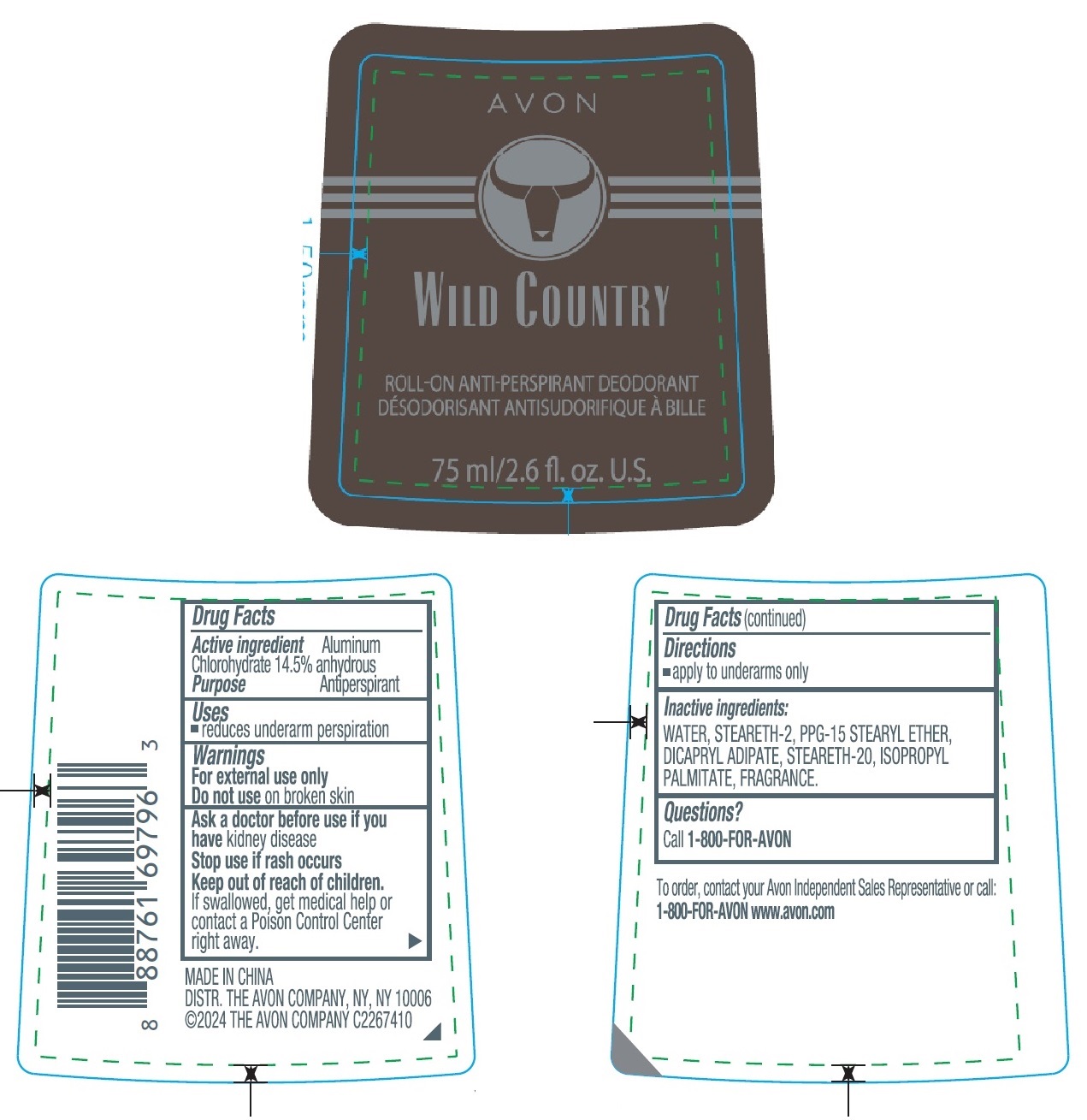
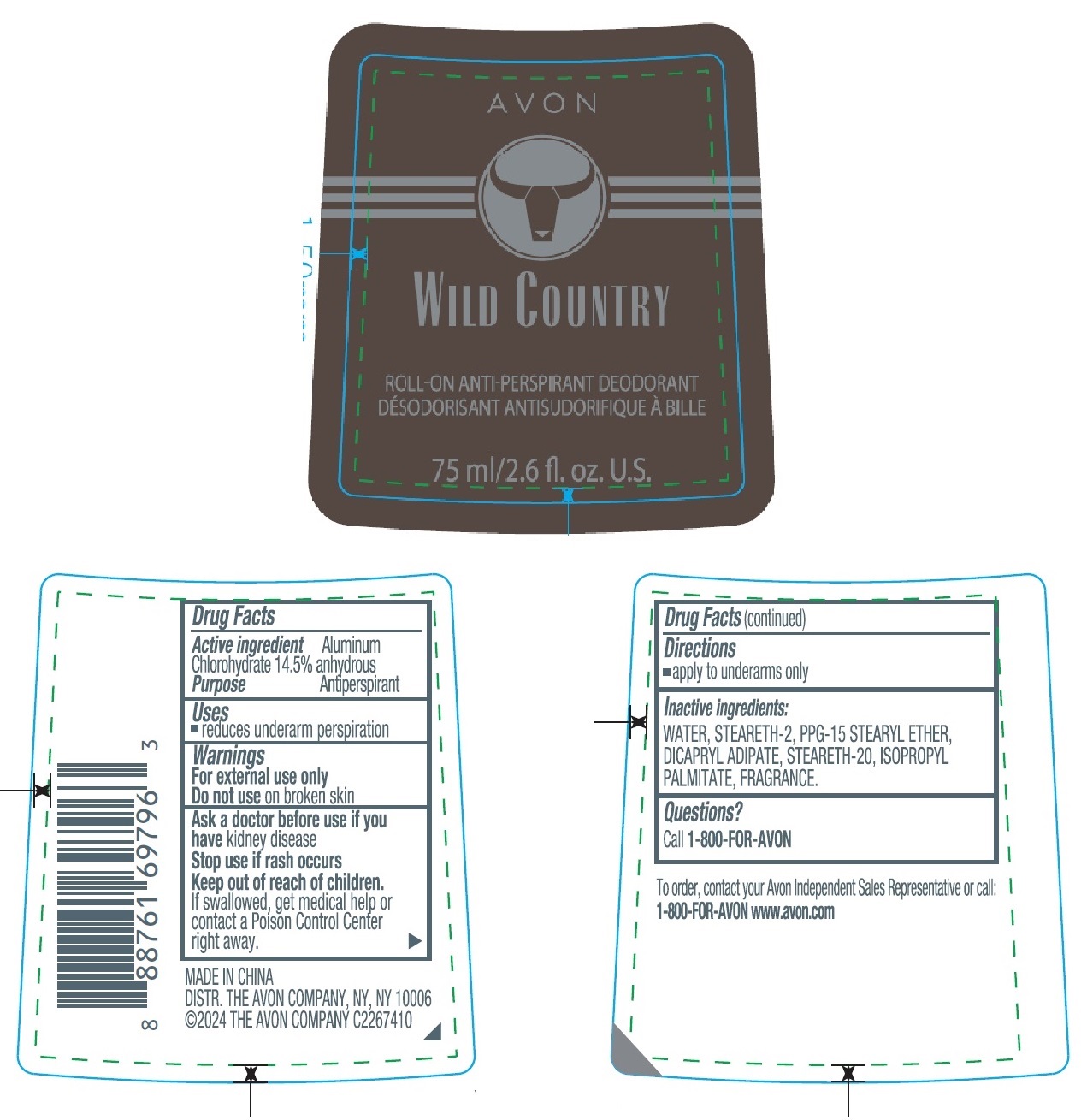
Label: AVON WILD COUNTRY ROLL-ON ANTI-PERSPIRANT DEODORANT- aluminum chlorohydrate stick
- NDC Code(s): 10096-2175-1
- Packager: The Avon Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
AVON WILD COUNTRY ROLL-ON ANTI-PERSPIRANT DEODORANT
aluminum chlorohydrate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10096-2175 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 145 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10096-2175-1 75 mL in 1 CANISTER; Type 0: Not a Combination Product 03/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 03/20/2024 Labeler - The Avon Company (080143520) Establishment Name Address ID/FEI Business Operations Avon Manufacturing (Guangzhou) Ltd. 544863277 manufacture(10096-2175)