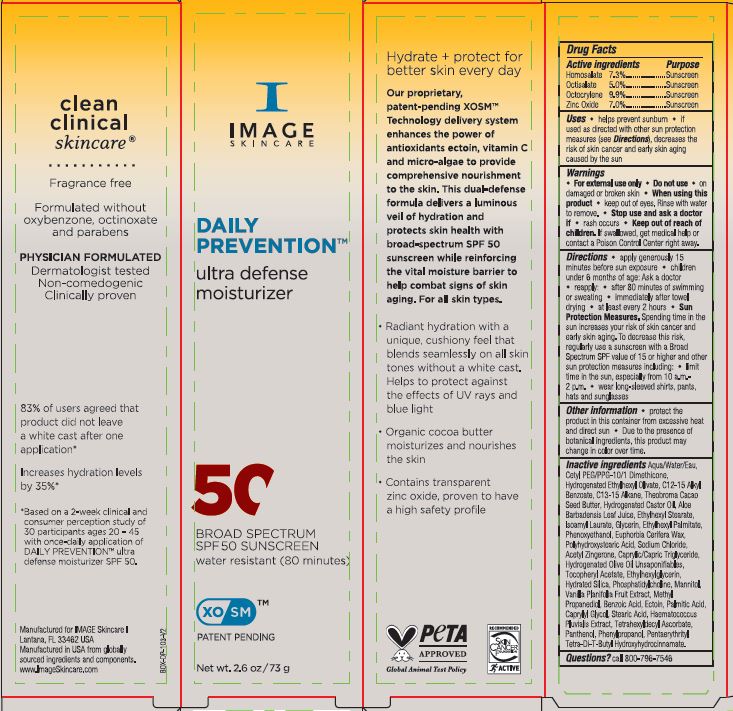
Label: DAILY PREVENTION ULTRA DEFENSE MOISTURIZER SPF 50- homosalate, octisalate,octocrylene and zinc oxide cream
- NDC Code(s): 62742-4255-1, 62742-4255-2, 62742-4255-3, 62742-4255-4
- Packager: Allure Labs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions • apply generously 15 minutes before sun exposure • children under 6 months of age: Ask a doctor • reapply: • after 80 minutes of swimming or sweating • immediately after towel drying • at least every 2 hours • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m.-2 p.m. • wear long-sleeved shirts, pants, hats and sunglasses
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients : Aqua/Water/Eau, Cetyl PEG/PPG-10/1 Dimethicone, Hydrogenated Ethylhexyl Olivete, C12-15 Alkyl Benzoate, C13-15 Alkane, Theobroma Cacao Seed Butter, Hydrogenated Castor Oil, Aloe Barbadensis Leaf Juice, Ethylhexyl Stearate, Isoamyl Laurate, Glycerin, Ethylhexyl Palmitate, Phenoxyethanol, Euphorbia Cerifera Wax, Polyhydroxystearic Acid, Sodium Chloride, Acetyl Zingerone, Caprylic/Capric Triglyceride, Hydrogenated Olive Oil Unsaponifiables, Tocopheryl Acetate, Ethylhexylglycerin, Hydrated Silica, Phosphatidylcholine, Mannitol, Vanilla Planifolia Fruit Extract, Methyl Propanediol, Benzoic Acid, Ectoin, Palmitic Acid, Caprylyl Glycol, Stearic Acid, Haematococcus Pluvialis Extract, Tetrahexyldecyl Ascorbate, Panthenol, Phenylpropanol, Pentaerythrityl Tetra-Di-T-Butyl Hydroxydrocinnamate.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAILY PREVENTION ULTRA DEFENSE MOISTURIZER SPF 50
homosalate, octisalate,octocrylene and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4255 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9.9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7.3 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL PEG/PPG-10/1 DIMETHICONE (HLB 4) (UNII: 8INO2K35FA) C13-15 ALKANE (UNII: 114P5I43UJ) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYL PALMITATE (UNII: 2865993309) SODIUM CHLORIDE (UNII: 451W47IQ8X) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BENZOIC ACID (UNII: 8SKN0B0MIM) ECTOINE (UNII: 7GXZ3858RY) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PHENYLPROPANOL (UNII: 0F897O3O4M) VANILLA (UNII: Q74T35078H) HYDRATED SILICA (UNII: Y6O7T4G8P9) PHOSPHATIDYLCHOLINE TRANSLOCATOR ABCB4 (UNII: 9EI49ZU76O) MANNITOL (UNII: 3OWL53L36A) WATER (UNII: 059QF0KO0R) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ACETYL ZINGERONE (UNII: V9D92S9YE5) STEARIC ACID (UNII: 4ELV7Z65AP) COCOA BUTTER (UNII: 512OYT1CRR) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) ISOAMYL LAURATE (UNII: M1SLX00M3M) PHENOXYETHANOL (UNII: HIE492ZZ3T) CANDELILLA WAX (UNII: WL0328HX19) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) HYDROGENATED OLIVE OIL UNSAPONIFIABLES (UNII: B8MIX97W95) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYDROGENATED ETHYLHEXYL OLIVATE (UNII: JBH5K4WD8L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PALMITIC ACID (UNII: 2V16EO95H1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4255-1 7 g in 1 TUBE; Type 0: Not a Combination Product 03/19/2024 2 NDC:62742-4255-3 1 in 1 CARTON 03/19/2024 2 NDC:62742-4255-2 73 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:62742-4255-4 142 g in 1 TUBE; Type 0: Not a Combination Product 03/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/19/2024 Labeler - Allure Labs (926831603) Registrant - Allure Labs (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs 926831603 manufacture(62742-4255)