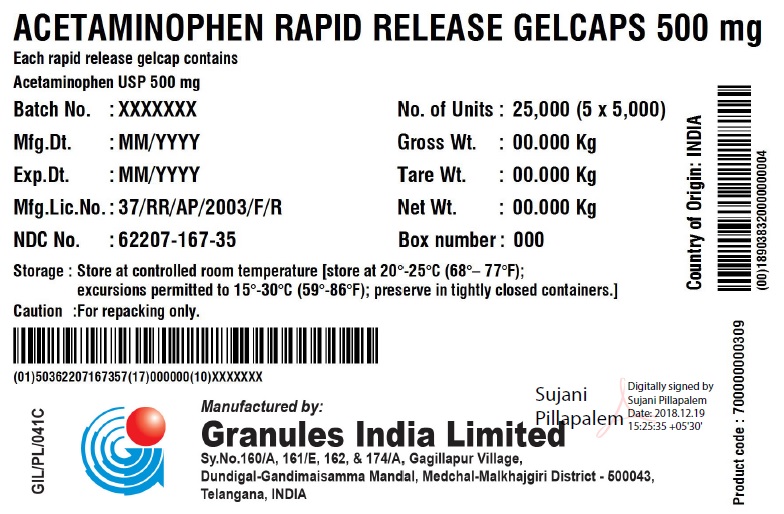
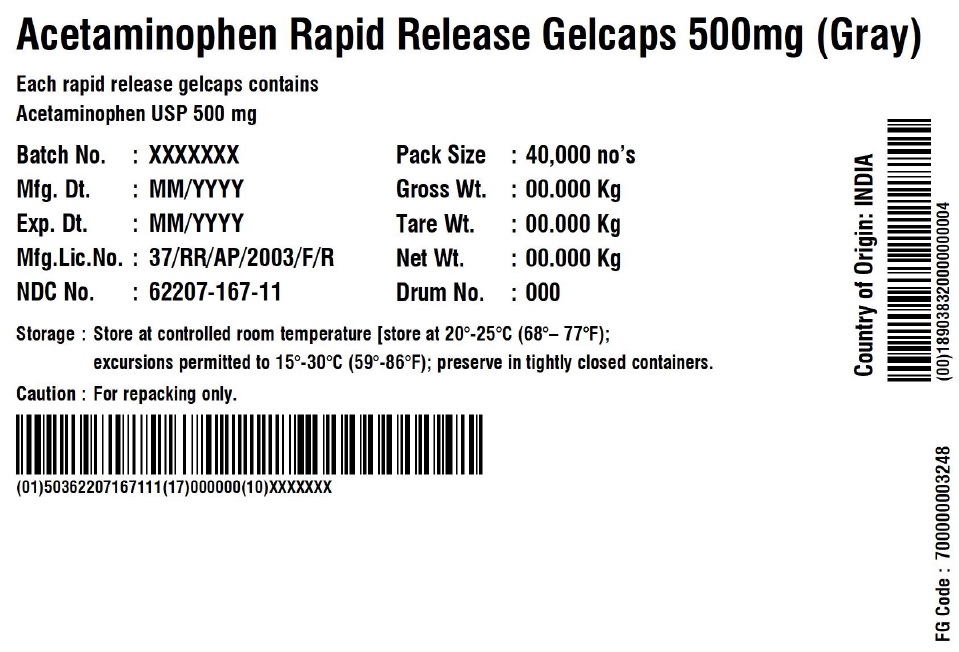
Label: ACETAMINOPHEN RAPID RELEASE- acetaminophen capsule, gelatin coated
-
NDC Code(s):
62207-167-11,
62207-167-21,
62207-167-22,
62207-167-31, view more62207-167-32, 62207-167-33, 62207-167-35
- Packager: Granules India Limited
- Category: BULK INGREDIENT
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
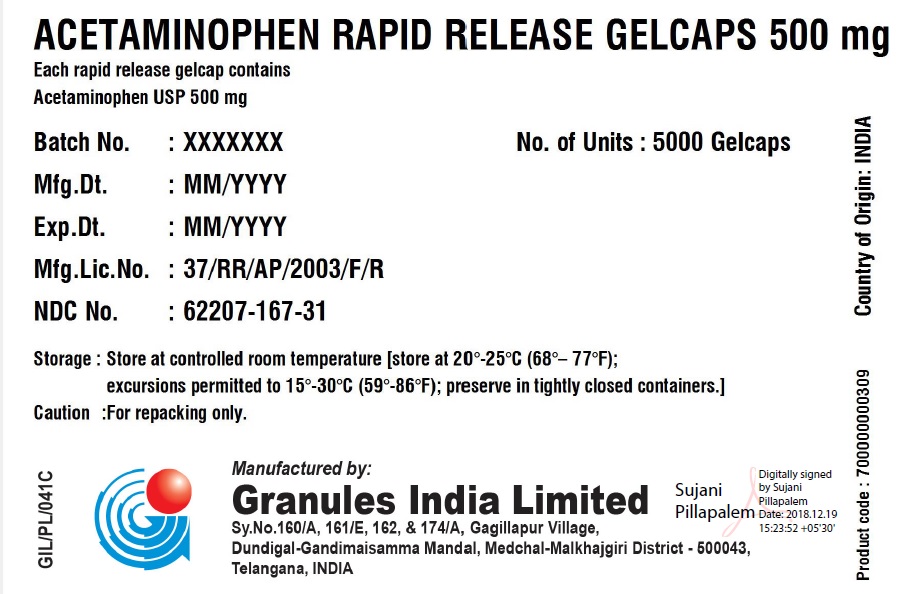
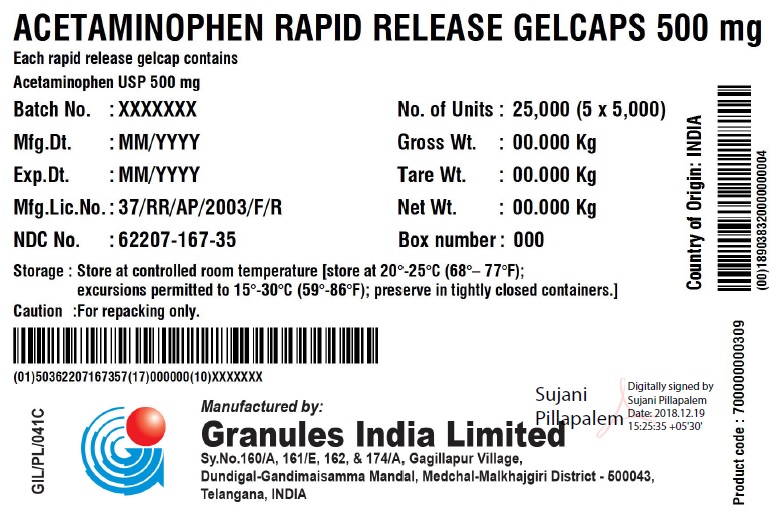
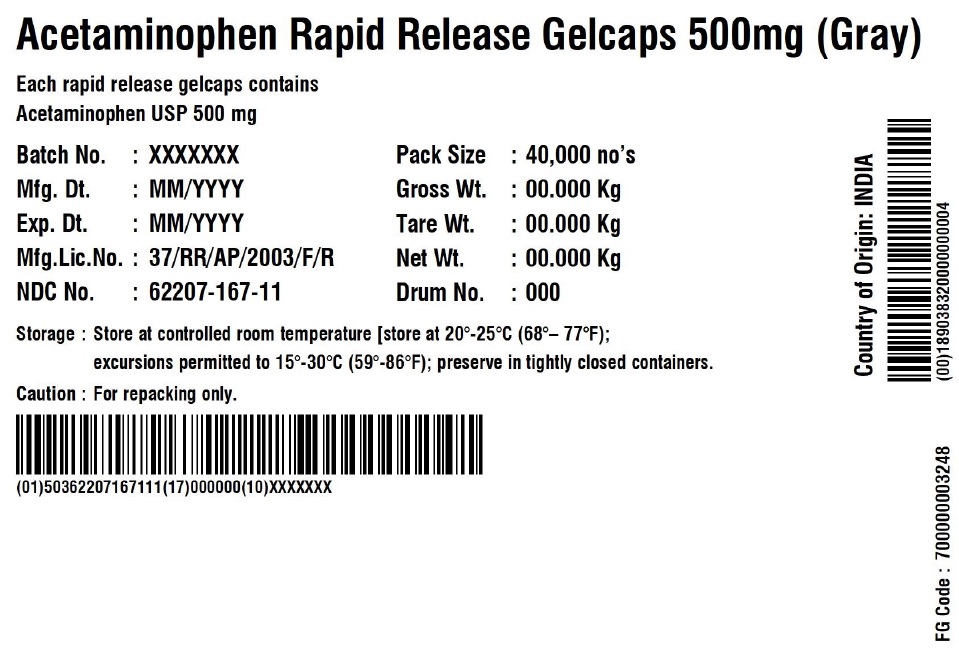
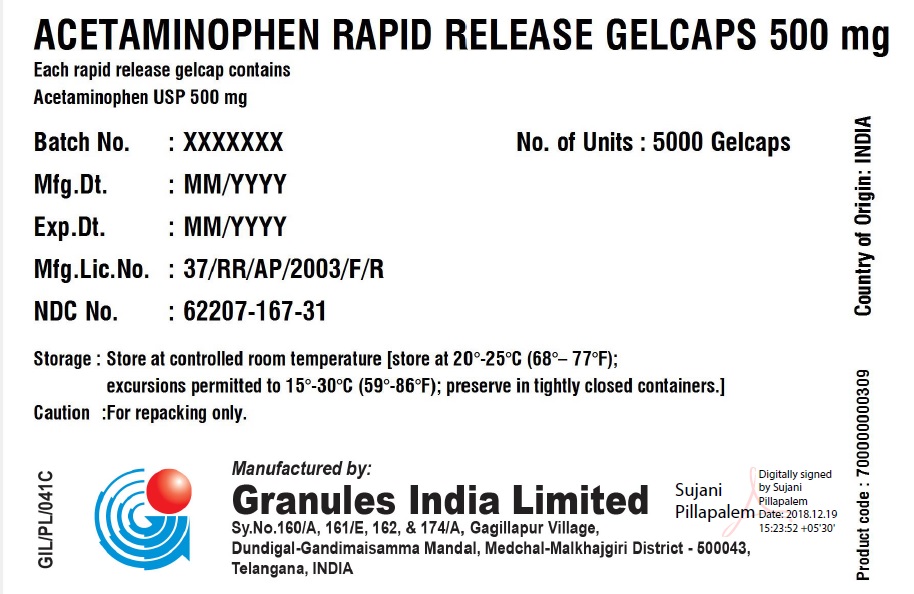
ACETAMINOPHEN RAPID RELEASE GELCAPS 500 MG
Each rapid release gelcap contains
Acetaminophen USP 500 mgBatch No.: XXX No. of Units 5 x 5000 Gelcaps Mfg. Dt.: MMM/YYYY Gross Wt.: 0.00 kg Exp. Dt.: MMM/YYYY Tare Wt.: 0.00 kg Mfg. Lic. No.: 37/RR/AP/2003/F/R Net Wt.: 0.00 kg NDC No. XXXXX-XXX-XX Box number: 000 Storage: Store at 20 – 25C (68 – 77F); excursions permitted to 15 – 30 C (59 – 86F); Preserve in tightly closed containers. Caution For Repacking only. Manufactured in India by:
Granules India Limited
Plot No. 160/A, 161/E, Gagillapur Village
Qutbullapur Mandal, Ranga Reddy Dt – 500 043, AP. - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN RAPID RELEASE
acetaminophen capsule, gelatin coatedProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:62207-167 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 499.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K30 (UNII: U725QWY32X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C RED NO. 33 (UNII: 9DBA0SBB0L) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) ALCOHOL (UNII: 3K9958V90M) Product Characteristics Color red, blue (blue-gray) Score no score Shape CAPSULE (banded two-part capsule) Size 19mm Flavor Imprint Code G;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-167-21 5000 in 1 BOX 10/29/2013 2 NDC:62207-167-22 25000 in 1 BOX 10/29/2013 3 NDC:62207-167-31 5000 in 1 POUCH 10/29/2013 4 NDC:62207-167-32 10000 in 1 POUCH 10/29/2013 5 NDC:62207-167-33 25000 in 1 POUCH 10/29/2013 6 NDC:62207-167-35 25000 in 1 BOX 10/29/2013 7 NDC:62207-167-11 39806 in 1 DRUM 05/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 10/29/2013 Labeler - Granules India Limited (915000087) Registrant - Granules India Limited (915000087) Establishment Name Address ID/FEI Business Operations Granules India Limited 918609236 manufacture(62207-167) , analysis(62207-167)