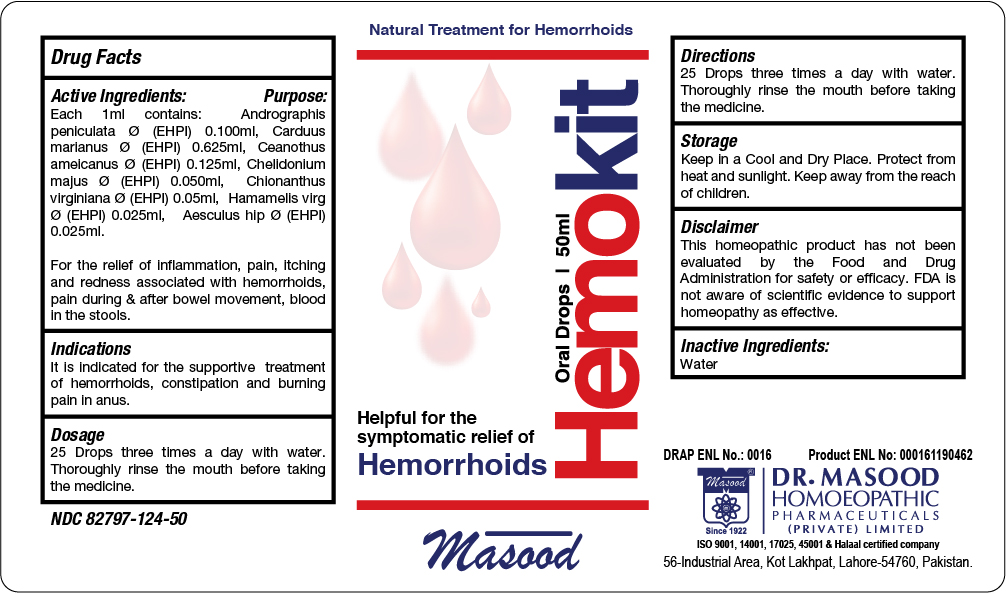
Label: HEMOKIT ORAL DROPS FOR HEMORRHOIDS- carduus marianus, hamamelis virgiana, ceanothus americanus, aesculus hip, andrographis paniculata, chionanthus virginiana , chelidonium majus liquid
- NDC Code(s): 82797-124-50
- Packager: Dr. Masood Homeopathic Pharmaceuticals Private Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Keep Out of Reach of Children
- Indications and Usage
- Dosage and Administration
- Storage
-
Active Ingredients
Active Ingredients Purpose
Aesculus hip: Haemorrhoids, with sharp shooting pains up the back
Andrographis peniculata Frequent urging for stool
Carduus marianus Haemorrhagic piles, prolapse of rectum, burning pain in anus and rectum
Ceanothus americanus Bearing down in abdomen and rectum
Chelidonium majus Burning and itching of anus.
Chionanthus virginiana Constipation
Hamamelis virgiana Haemorrhoids, bleeding profusely, with soreness
- INACTIVE INGREDIENT
- Warnings
- Warnungs:
-
Purpose
Assists in temporarily relief of following symptoms:
Haemorrhoids, with sharp shooting pains up the back
Frequent urging for stool
Haemorrhagic piles, prolapse of rectum, burning pain in anus and rectum
Bearing down in abdomen and rectum
Burning and itching of anus.
Constipation
Haemorrhoids, bleeding profusely, with soreness
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMOKIT ORAL DROPS FOR HEMORRHOIDS
carduus marianus, hamamelis virgiana, ceanothus americanus, aesculus hip, andrographis paniculata, chionanthus virginiana , chelidonium majus liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82797-124 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHELIDONIUM MAJUS WHOLE (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS WHOLE - UNII:7E889U5RNN) CHELIDONIUM MAJUS WHOLE 0.05 mg in 1 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 0.025 mg in 1 mL ANDROGRAPHIS PANICULATA WHOLE (UNII: 0P49L952WZ) (ANDROGRAPHIS PANICULATA WHOLE - UNII:0P49L952WZ) ANDROGRAPHIS PANICULATA WHOLE 0.1 mg in 1 mL MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 0.625 mg in 1 mL AESCULUS HIPPOCASTANUM BARK (UNII: 7U76MXL14N) (AESCULUS HIPPOCASTANUM BARK - UNII:7U76MXL14N) AESCULUS HIPPOCASTANUM BARK 0.025 mg in 1 mL CEANOTHUS AMERICANUS WHOLE (UNII: 8AD0I300BR) (CEANOTHUS AMERICANUS WHOLE - UNII:8AD0I300BR) CEANOTHUS AMERICANUS WHOLE 0.125 mg in 1 mL CHIONANTHUS VIRGINICUS WHOLE (UNII: 848H67V9N5) (CHIONANTHUS VIRGINICUS WHOLE - UNII:848H67V9N5) CHIONANTHUS VIRGINICUS WHOLE 0.05 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82797-124-50 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/19/2024 Labeler - Dr. Masood Homeopathic Pharmaceuticals Private Limited (645453119) Registrant - Dr. Masood homeopathic Pharmaceuticals Private Limited (645453119) Establishment Name Address ID/FEI Business Operations Dr. Masood Homeopathic Pharmaceuticals Private Limited 645453119 manufacture(82797-124) , pack(82797-124) , label(82797-124)