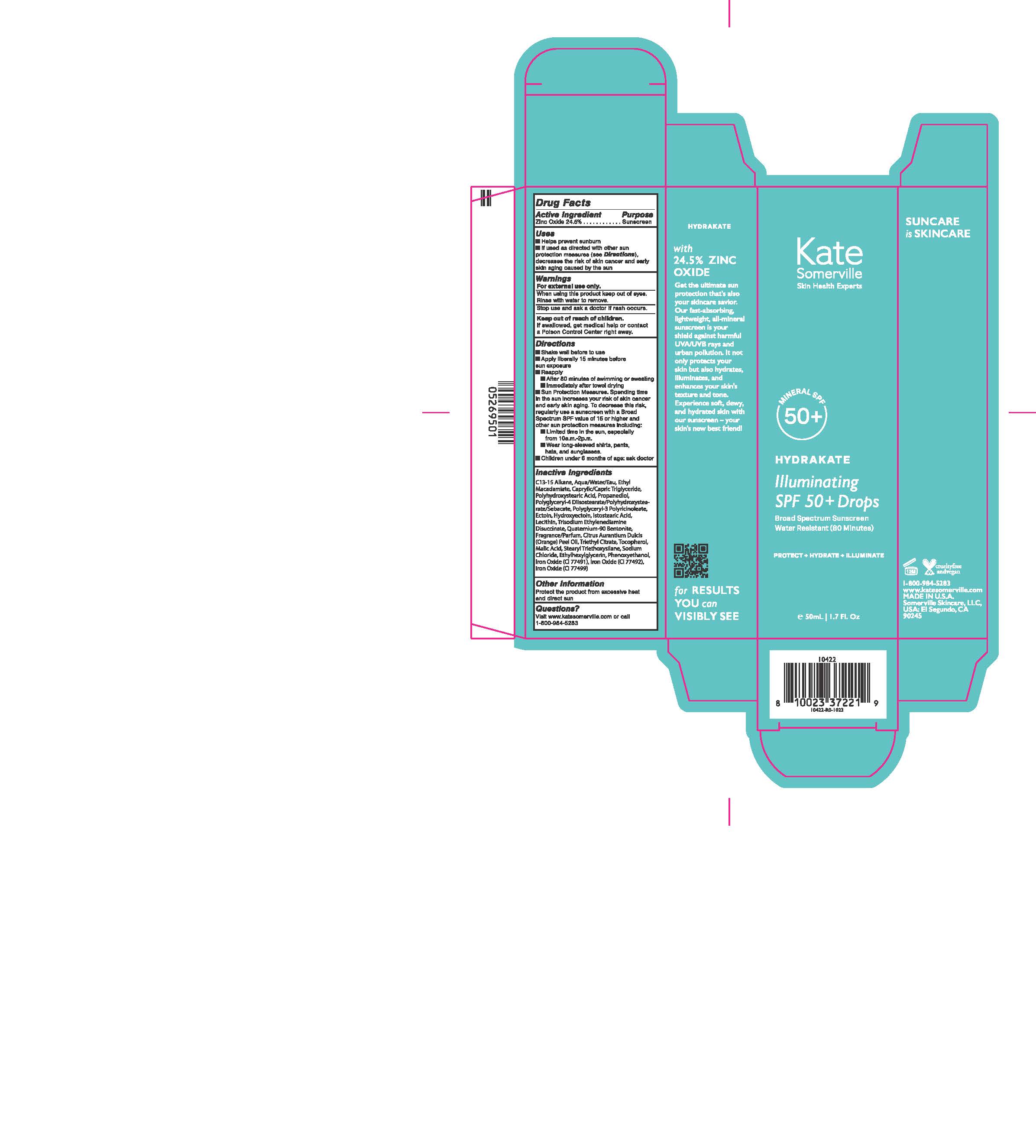
Label: ILLUMINATING SPF 50 PLUS DROPS BROAD SPECTRUM SUNSCREEN- zinc oxide lotion
- NDC Code(s): 56152-0253-1
- Packager: Cosmetic Enterprise Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- Shake well before use
- Apply liberally 15 minutes before sun exposure
- Reapply
- After 80 minutes of swimming or sweating
- immediately after towel drying
- Sun Protection Meaures. Spending time under the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regurlarly use a sunscreen with an SPF of 15 or higher, and other sun protection meaures, including:
- limit time under the sun, especially from 10:00 a.m. to 2:00 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months of age: ask doctor
-
INACTIVE INGREDIENT
Inactive Ingredients
C13-15 Alkane, Aqua/water/Eau, Ethyl Macadamiate, Caprylic/Capric Triglyceride, Polyhydroxystearic Acid, Propanediol, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Polyglyceryl-3 Polyricinoleate, Ectoin, Hydroxyectoin, Isostearic acid, Lecithin, Trisodium Ethylenediamine Disuccinate, Quaternium-90 Bentonite, Fragrance/Parfum, Citrus Aurantium Dulcis (Orange) Peel Oil, Triethyl Citrate, Tocopherol, Malic Acid, Stearyl Triethoxysilane, Sodium Chloride, Ethylhexylglycerin, Phenoxyethanol, Iron Oxide (CI 77491), Iron Oxide (CI 77492), Iron Oxide (CI 77499)
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ILLUMINATING SPF 50 PLUS DROPS BROAD SPECTRUM SUNSCREEN
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56152-0253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 24.5 g in 100 mL Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ECTOINE (UNII: 7GXZ3858RY) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ISOSTEARIC ACID (UNII: X33R8U0062) ETHYL MACADAMIATE (UNII: ANA2NCS6V1) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE RED (UNII: 1K09F3G675) SODIUM CHLORIDE (UNII: 451W47IQ8X) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) STEARYL TRIETHOXYSILANE (UNII: 1VN9P25L8H) C13-15 ALKANE (UNII: 114P5I43UJ) WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) HYDROXYECTOIN (UNII: CIJ7YN252E) MALIC ACID (UNII: 817L1N4CKP) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56152-0253-1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/22/2024 Labeler - Cosmetic Enterprise Ltd (017701475)