Label: VACANT HYPROMELLOSE- vacant hypromellose capsules capsule
- NDC Code(s): 84175-003-01
- Packager: Qinhuangdao Medicinal Capsule Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated March 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
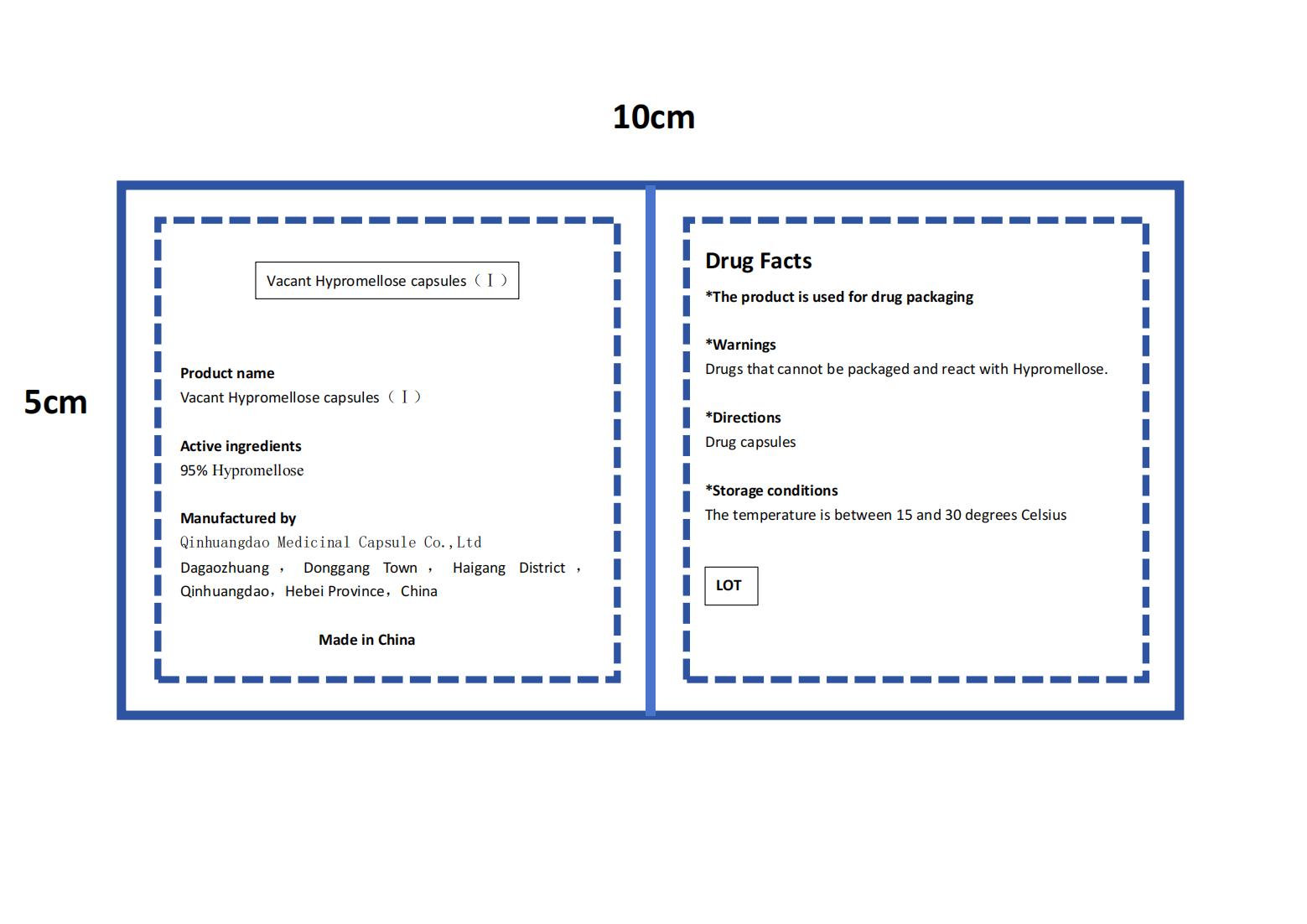
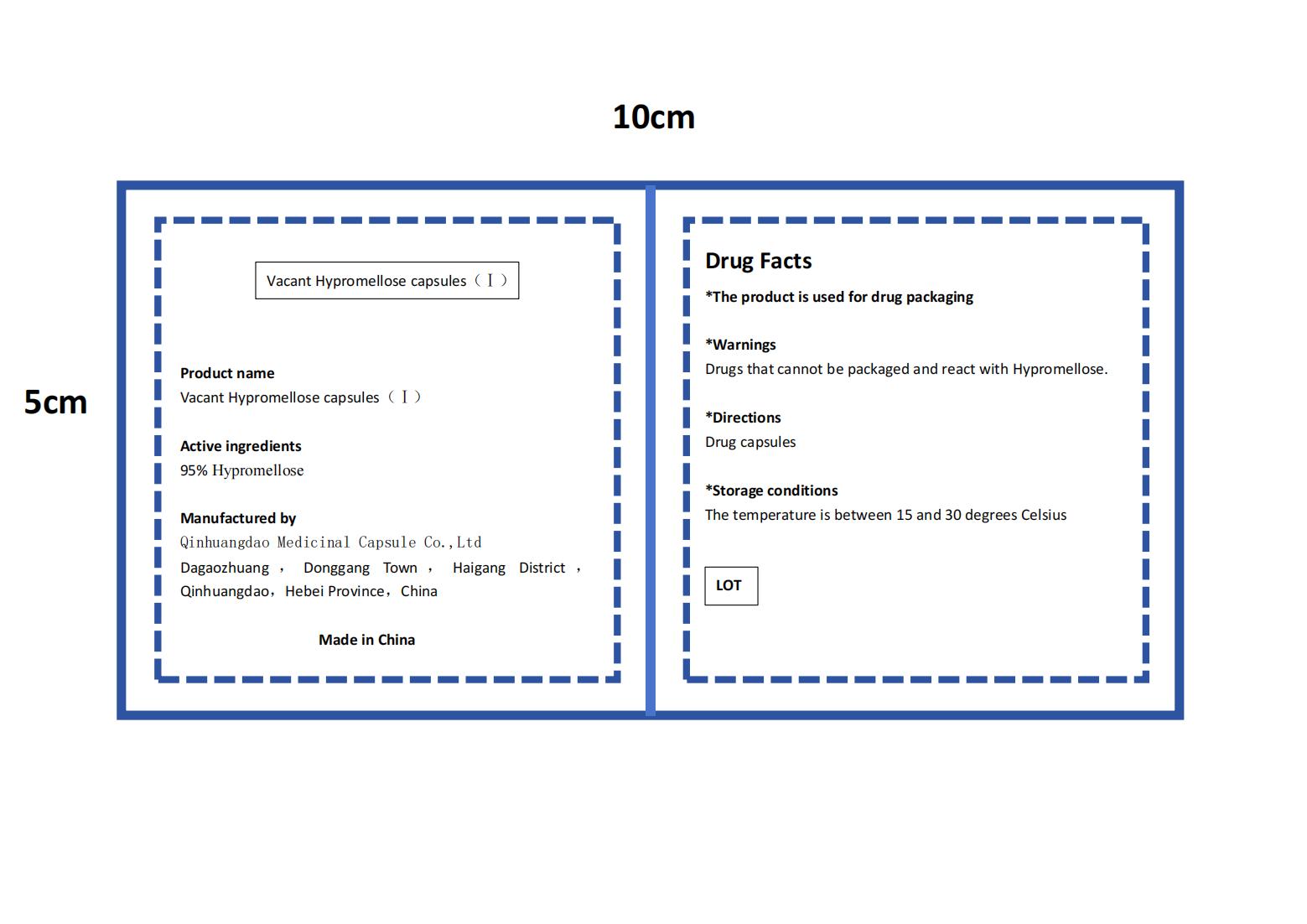
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VACANT HYPROMELLOSE
vacant hypromellose capsules capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84175-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPROMELLOSES (UNII: 3NXW29V3WO) (HYPROMELLOSES - UNII:3NXW29V3WO) HYPROMELLOSES 0.95 g in 1 g Inactive Ingredients Ingredient Name Strength .KAPPA.-CARRAGEENAN (UNII: DV181E4639) 0.05 g in 1 g Product Characteristics Color white Score no score Shape BULLET Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84175-003-01 100 g in 1 CARTON; Type 0: Not a Combination Product 03/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 03/18/2024 Labeler - Qinhuangdao Medicinal Capsule Co.,Ltd (547622519) Registrant - Qinhuangdao Medicinal Capsule Co.,Ltd (547622519) Establishment Name Address ID/FEI Business Operations Qinhuangdao Medicinal Capsule Co.,Ltd 547622519 manufacture(84175-003)